Abstract
In this study, we investigated the impact of endogenous hydrogen sulfide (H2S) on toll-like receptors (TLRs)-mediated inflammatory response and apoptosis in renal ischemia–reperfusion injury (IRI). Twenty-four male Wistar rats were randomly divided into four groups: sham, IR, IR + propargylglycine (PAG) and IR + hydroxylamine (HA). After right nephrectomy, rats were given saline for the sham and IR group, PAG for the IR + PAG group and HA for the IR + HA group, through the left renal artery for 20 min. Five minutes after drug administration, all rats except sham underwent 45 min of left renal ischemia followed by 24 h of reperfusion. Kidneys were harvested for histological and biochemical evaluation. Levels of TLRs, downstream signaling molecules and pro-inflammatory cytokines were determined by Western blot or immunohistochemistry. Hematoxylin and eosin (H&E) stained renal sections were used for histological grading of renal injury. Apoptotic cells were detected by TUNEL assay. Compared to the sham group, rats in the IR group showed higher renal levels of TLR-2, TLR-4, nuclear NF-κB p65, phosphorylated ASK1, phosphorylated TRAF2, IL-1β, IL-6, IL-18 and TNF-α (p < 0.05), and exhibited acute kidney injury (p < 0.05) and apoptosis (p < 0.05). Compared to the IR group, rats receiving PAG or HA showed significantly higher levels of TLR-2, TLR-4, nuclear NF-κB p65, phosphorylated ASK1, phosphorylated TRAF2, IL-1β, IL-6, IL-18 and TNF-α (p < 0.01), more severe acute kidney injury (p < 0.05) and increased apoptosis (p < 0.01). Thus, inflammatory response and apoptosis mediated by TLRs are involved in renal IRI. Inhibition of endogenous H2S significantly activated inflammatory response and apoptosis, and thus promoted renal IRI.
Introduction
Kidney is a high-perfusion organ and is sensitive to ischemia and ischemia–reperfusion (IR). Renal ischemia–reperfusion injury (IRI) often happens in clinical conditions such as hypovolemic shock, kidney transplantation, partial nephrectomy and vascular surgery.Citation1,Citation2 Operative interruption of renal blood flow during transplantation can lead to IRI, which may cause acute kidney injury (AKI) and affect recovery of early graft function and graft survival.Citation3 Thus, it is of great importance to investigate how to prevent and treat IRI induced acute/chronic renal disease.
Hydrogen sulfide (H2S) is considered to be the third endogenous gasotransmitter following nitric oxide and carbon monoxide.Citation4 Endogenous H2S is mainly produced by cysthionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE)-catalyzed conversion of cysteine. The distribution of endogenous H2S producing enzymes is tissue specific: the nerve system mainly expresses CBS; the cardiovascular system mainly expresses CSE; kidney, liver and pancreas express both enzymes.Citation4–6 Endogenous H2S exists in the form of H2S (1/3) and NaHS (2/3).Citation7 NaHS dissociates to Na+ and HS−, and then HS− associates with H+ to produce H2S.Citation8
H2S has been considered as a critical mediator of multiple physiological processes. H2S can relax smooth muscle, protect blood vessel through inhibition of vascular remodeling, be involved in cell proliferation and apoptosis, modulate neuronal activity and regulate endocrine function.Citation4,Citation9–12 Studies have shown that H2S can ameliorate IRI in the heart, lung, liver, kidney and small intestine, and thus plays a general protective role against IR in different organs.Citation13–17 In a rat renal IRI model, H2S level was significantly decreased; however, administration of the exogenous H2S donor, NaHS, ameliorated IRI and improved renal function.Citation18 Similar observation has been obtained in living mice.Citation19 In a bilateral renal IR model using Wistar rats, administration of CSE inhibitor PAG impaired recovery of renal function with increased serum creatinine and urea after 45-min ischemia and 72-h reperfusion.Citation17 After 30 min of reperfusion, pretreatment of exogenous H2S significantly decreased the phosphorylation of mitogen-activated protein kinases (p-38, c-JUN N-terminal kinase 1/2 and extracellular signal-regulated kinase 1/2). After 60 min of reperfusion, exogenous H2S reduced acute tubular necrosis, increased the expression of Bcl-2 and prevented the nuclear translocation of the NF-κB p65 subunit. These findings demonstrated that H2S played a protective role against IR-induced renal injury and dysfunction, which might be due to both anti-apoptotic and anti-inflammatory effects.Citation17
Toll-like receptors (TLRs) are an important family of pattern recognition receptors that play essential roles in inflammatory response of the innate immunity. TLRs are expressed in many types of immune cells including lymphocytes, macrophages, neutrophils, endothelial and epithelial cells and so on. Based on their cellular localization, TLRs fall into two categories: cell membrane-localized TLRs (TLR-1, −2, −4, −5, −6, −10, −11, −12 and −13) and intracellular TLRs (TLR-3, −7, −8 and −9).Citation20 The family members, TLR-2 and TLR-4, are constitutively expressed in renal tubular epithelial cells.Citation21 They are activated in renal IRI and induce kidney inflammation and apoptosis, which may cause AKI.Citation21–22
Therefore, the aim of this study was to investigate the impact of endogenous H2S on TLR pathways in renal IRI in rats. We first established a rat renal IRI model which was pre-treated with CSE inhibitor propargylglycine (PAG) or CBS inhibitor hydroxylamine (HA). We then examined whether inhibition of endogenous H2S could promote TLR-mediated inflammatory response and apoptosis to investigate the protective mechanism of H2S against rat renal IRI.
Materials and methods
Animals and surgery procedure
The project was approved by the experimental ethics committee of Shanxi Medical University. Twenty-four male Wistar rats, weighing 180–220 g, were obtained from the animal center of Shanxi Medical University. Animals were fed with standard rat chow and water ad libitum and housed in cages with controlled temperature.
Rats were randomly divided into four groups (n = 6 in each group): sham, IR, IR + PAG and IR + HA. Following a 12-h fasting period, the animals were anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g, Tianjin Guangfu Fine Chemicals, Tianjin, China). After the abdomen was opened through a midline incision, both renal pedicles were exposed. After right nephrectomy, rats were given (i) saline for sham and IR group, (ii) PAG (2 μmol/min, Sigma-Aldrich, St. Louis, MO) for IR + PAG group, (iii) HA (300 nmol/min, Sigma-Aldrich, St. Louis, MO) for IR + HA group, through the left renal artery for 20 min. Five minutes after drug administration, all rats except sham underwent 45 min of left renal ischemia followed by 24 h of reperfusion. After reperfusion, kidneys were harvested for histological and biochemical examination.
Protein extraction
Total protein
From liquid nitrogen-preserved kidneys, 30 mg of tissue samples were homogenized in lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 µg/mL Aprotinin, 5 µg/mL Leupeptin, 1% Triton x-100, 1% sodium deoxycholate, 0.1% SDS) and centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatants were collected, subjected to BCA assay (Beyotime, Shanghai, China) for determination of protein concentration and used for Western blot analysis.
Nuclear protein
Twenty milligrams of fresh tissue samples were homogenized in 800 mL (1:40, w/v) lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.05% NP40, pH 7.9) and incubated on ice for 10 min. The supernatants were collected and centrifuged at 3000g for 10 min at 4 °C. The pellets were re-suspended in buffer (300 mM NaCl, 5 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 26% glycerol (v/v), pH 7.9), incubated on ice for 30 min and centrifuged at 24,000 g for 20 min at 4 °C. The supernatants were collected, subjected to BCA assay for determination of protein concentration and used for Western blot analysis.
Western blot analysis
Thirty micrograms of each protein sample were denatured, separated by SDS-PAGE and transferred onto a PVDF membrane (Sigma-Aldrich). The blot was blocked in 5% skim milk for 2 h, and incubated with rabbit anti-rat TLR2 (#13744), TLR4 (#2219), NF-κB P65 (#4764), phospho-ASK1 (Ser967, #3764) or phospho-TRAF 2 antibodies (Ser11, #13908, 1:1000, Cell Signaling Technology, Danvers, MA) at 4 °C overnight. After incubation with HRP-conjugated goat anti-rabbit (1:2000, Bioss, Beijing, China) antibody at room temperature for 1 h, protein bands were detected by ECL Chemiluminescent (Bogoo, Shanghai, China). β-Actin (1:2000, Bioss, Beijing, China) served as an internal control for semi-quantification of the proteins of interest.
Renal histological examination
Kidneys were fixed in 10% formalin (Eysin, Shanghai, China). After dehydration using graded ethanol, pieces of kidney were embedded in paraffin, subsequently cut into 3-μm sections and stained with hematoxylin and eosin (H&E, Beyotime, Shanghai, China). The histology was assessed by two pathologists blinded to the samples. The sections were scored with a scale designed to evaluate the degree of renal tubulointerstitial damages, such as renal epithelial cell degeneration, necrosis, brush border loss, tube formation and inflammatory cell infiltration. The scoring system used was 0 (none), 1 (<10%), 2 (11–25%), 3 (26–45%), 4 (46–75%), 5 (>76%). A minimum of 10 fields (200 × magnification) of renal cortex–medulla junction for each kidney section were examined and assigned for severity of changes.
Immunohistochemical staining of pro-inflammatory cytokines
Kidney tissues were embedded in paraffin and cut into 3 -μm sections. The sections were deparaffinized with dimethylbenzene, rehydrated and incubated with 3% hydrogen peroxide at room temperature for 10 min. After PBS wash for 5 min and microwave-antigen retrieval, the sections were blocked by goat serum (Bioss) at room temperature for 10 min, and incubated with rabbit anti-rat IL-1β, IL-6, IL-18 or TNF-α (1:200, Bioss) at 4 °C overnight. The sections were incubated with biotinylated goat anti-rabbit (1:1,000, Bioss) antibody at 37 °C for 30 min and then with streptavidin–biotin complex (Boster, Wuhan, China) at 37 °C for 30 min. Immunostaining was visualized with diaminobenzidine buffer (Boster, Wuhan, China), counterstained with hematoxylin (Beyotime, Shanghai, China) and then analyzed using JD-801 morphological image analysis system (Jeda, Nanjing, Jiangsu, China). Six fields (200 × magnification) of each section were examined and protein levels were assessed by mean grey values.
Apoptosis TUNEL assay
Kidneys were fixed in 10% formalin for 24 h. After dehydration using graded ethanol, pieces of kidney were embedded in paraffin, subsequently cut into 5-μm sections and stained using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick and labeling) assay kit (Roche, Basel, Switzerland) according to the manufacturer’s instruction. TUNEL positive cells were analyzed under a light microscope (Olympus, Tokyo, Japan). For each section, six non-overlapping fields were analyzed (100 × magnification).
Statistical analysis
All values were expressed as mean ± standard error of the mean (SEM). All statistical analyses were performed using SPSS release 16.0 (SPSS, Chicago, IL). For the data from Western blot analysis, immunohistochemistry and TUNEL assay, comparisons between groups were evaluated with one-way ANOVA and comparisons between means were evaluated with Fisher’s Least Significant Difference (LSD) test. For the data from histological examination, comparisons between groups were evaluated with Mann–Whitney U test. Statistical significance was accepted a value of p < 0.05.
Results
Expression of toll-like receptors in kidney after IRI
There was a significant increase in the TLR-2 and −4 levels in the IR group compared to the sham group (p < 0.05; see ). Intake of PAG or HA prior to IRI produced more significant increases in the TLR-2 and −4 levels in the IR + PAG and IR + HA group compared to the IR group (p < 0.01; see ).
Figure 1. Expression of toll-like receptors in kidney after IRI. (A) Western blot analysis of TLR-2 and −4 in kidney after IRI. β-Actin served as internal control for semiquantification of TLR-2 and −4. Relative protein levels of TLR-2 (B) and TLR-4 (C) were compared among different groups. Notes: Data shown are mean ± SEM; *statistically significant from sham group (p < 0.05); γstatistically significant from IR group (p < 0.01); n = 3 each.
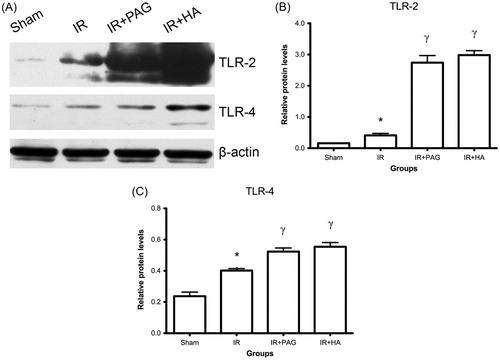
Activation of nuclear NF-κB p65, phosphorylated ASK1 and phosphorylated TRAF2 in kidney after IRI
Compared to the sham group, levels of nuclear NF-κB p65, phosphorylated ASK1 and TRAF2 were significantly increased in the IR group (p < 0.05; see ). Nuclear NF-κB p65, phosphorylated ASK1 and TRAF2 levels were significantly higher in rats receiving PAG or HA than the IR group (p < 0.01; see ).
Figure 2. Increase of nuclear NF-κB p65, phosphorylated ASK1 and phosphorylated TRAF2 in kidney after IRI. (A) Western blot analysis of NF-κB p65, phosphorylated-ASK1 and TRAF2 in kidney after IRI. β-Actin served as internal control for semi-quantification of NF-κB p65, phosphorylated-ASK1 and TRAF2. Relative protein levels of NF-κB p65 (B), phosphorylated-TRAF2 (C) and ASK1 (D) were compared among different groups. Notes: Data shown are mean ± SEM; *statistically significant from sham group (p < 0.05); γstatistically significant from IR group (p < 0.01); n = 3 each.
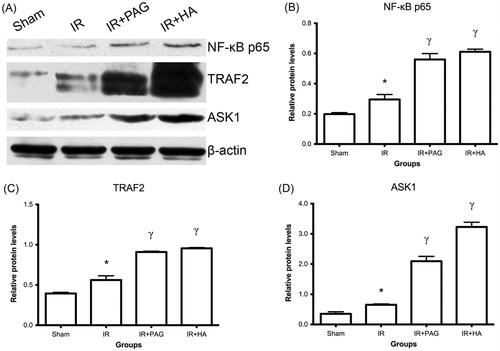
Histological evaluation
Histological grading of renal injury is displayed in .
Table 1. Histological evaluation of kidney sections for each group.
Compared to the sham group, the total injury score was significantly increased in the renal IR group (p < 0.05). Total histological injury scores were further increased in rats receiving PAG or HA, indicating significant tubular injury (p < 0.05). Representative histological samples from all groups are shown in .
Figure 3. Histological evaluation of kidneys after IRI. (A) Representative histological photographs of kidney tissues from different groups. (B) Detailed histological scores of each group. Notes: Data shown are individual values and medians; *statistically significant from sham group (p < 0.05); γstatistically significant from IR group (p < 0.05); n = 6 each.
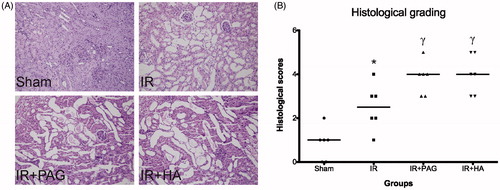
Expression of pro-inflammatory cytokines in kidney after IRI
Immunohistochemical data showed that levels of IL-1β, IL-6, IL-18 and TNF-α were low in the sham group while those levels were significantly higher in the IR group than those in the sham group (p < 0.01; see ). Levels of IL-1β, IL-6, IL-18 and TNF-α were even higher in rats receiving PAG or HA than the IR group (p < 0.01; see ).
Figure 4. Expression of pro-inflammatory cytokines in kidney after IRI. Immunohistochemical analysis of IL-1β (A), IL-6 (B), IL-18 (C) and TNF-α (D) in kidney after IRI. Levels of IL-1β (E), IL-6 (F), IL-18 (G) and TNF (H) were compared among different groups. Notes: Data shown are mean ± SEM; *statistically significant from sham group (p < 0.01); γstatistically significant from IR group (p < 0.01); n = 6 each.
Apoptosis in kidney after IRI
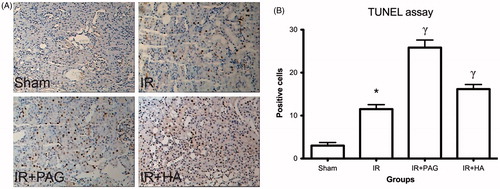
TUNEL assay detected very few apoptotic cells in the sham group but significantly more in the IR group (p < 0.01; see ). In rats receiving PAG or HA, apoptotic cells were significantly increased compared to those in the IR group (p < 0.05; see ).
Figure 5. Apoptosis in kidney after IRI. (A) Representative photographs of TUNEL-stained kidney tissues from different groups. (B) Numbers of apoptotic cells were estimated and compared among different groups. Notes: Data shown are mean ± SEM; *statistically significant from sham group (p < 0.01); γstatistically significant from IR group (p < 0.05); n = 6 each.
Discussion
TLRs play critical roles in inflammatory responses caused by innate immunity. It has been found that during IRI-induced AKI, TLR-2 and TLR-4 are up-regulated and activated in renal tubular epithelial cells, which activates intracellular signaling pathways through NF-κB or AP-1. Subsequently up-regulated mediators such as IL-1β, IL-6, IL-18, MCP1, IFN-γ and TNF-α induce inflammatory responses, leading to acute tubulointerstitial injury.Citation20,Citation22,Citation23 In our study, rat kidneys taken after ischemia–reperfusion showed significant renal pathological injury with elevated levels of TLR-2, TLR-4 and activated NF-κB, as well as increased expression of inflammatory factors (IL-1β, IL-6, IL-18 and TNF-α). Moreover, rats, of which the endogenous H2S was inhibited by PAG or HA, showed more severe renal pathological injury and further increased levels of TLR-2, TLR-4, activated NF-κB and the aforementioned inflammatory factors. These findings indicate that H2S may suppress renal IRI-induced inflammatory response possibly through inhibiting the TLR-2/4 pathway. However, it is worth noting that there are other stimuli for TNF-α production. For instance, oxidative stress during IR may stimulate transcription factors involved in TNF-α expression.Citation24 TNF-α is able to induce its own production. It has been reported that at low level, H2S protects organs from oxidative stress.Citation25 Thus, the increase in TNF-α production caused by H2S inhibition might not be purely due to TLR-2/4 up-regulation.
Studies have shown that renal IRI in mice causes up-regulation of TLR-4 in renal tubules which in turn mediates the elevated expression of TRAF2 and the activation of ASK1 and JUN N-terminal kinase 1, leading to cell apoptosis.Citation21 In our study, rat kidneys taken after ischemia–reperfusion showed significantly increased levels of TLR-4, TRAF2 and phosphorylated ASK1, which was associated with renal pathological injury and cell apoptosis. These effects were further enhanced in H2S inhibitor pre-treated rats. Thus, H2S can prevent activation of ASK1 and apoptosis of renal tubule cells through down-regulation of TLR4 and TRAF2.
The pyridoxal-5′-phosphate (PLP)-dependent enzymes CSE and CBS are two major enzymes for the production of endogenous H2S.Citation26 In this study, PAG and HA were used to suppress H2S production through the inhibition of CSE and CBS. PAG is a selective inhibitor for CSE, while HA has broad biological activity.Citation26 HA inhibits not only CSE and CBS, but also other PLP enzymes. In addition, HA is capable of generating nitric oxide. Thus, studies using HA as H2S inhibitor must be interpreted with caution. In this study, the decrease of endogenous H2S after HA treatment was confirmed by methylene blue method (data not shown). Moreover, the effects of PAG, a specific inhibitor of CSE, on inflammatory signaling and apoptosis were comparable. Thus, the actions of HA is most likely due to the inhibition of H2S production and not its other actions.
In conclusion, our study demonstrated that endogenous H2S plays a protective role in rat renal IRI by suppressing inflammatory response and cell apoptosis in kidney likely through inhibiting the activation of TLR-2/4. Agents that release H2S may potentially be applied in clinical conditions with renal IRI.
Acknowledgments
We thank Dr. Chen Wang and Dr. Lifang Gao for their help in histological analyses.
Declaration of interest
The authors report no conflicts of interest.
References
- Inal M, Altinisik M, Bilgin MD. The effect of quercetin on renal ischemia and reperfusion injury in the rat. Cell Biochem Funct. 2002;20(4):291–296
- Abreu Lde A, Kawano PR, Yamamoto H, Damiao R, Fugita OE. Comparative study between trimetazidine and ice slush hypothermia in protection against renal ischemia/reperfusion injury in a porcine model. Int Braz J Urol. 2011;37(5):649–656
- Hunter JP, Hosgood SA, Patel M, et al. Effects of hydrogen sulphide in an experimental model of renal ischemia–reperfusion injury. Br J Surg. 2012;99(12):1665–1671
- Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–1798
- Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5(4):493–501
- Yamamoto J, Sato W, Kosugi T, et al. Distribution of hydrogen sulfide (H(2)S)-producing enzymes and the roles of the H(2)S donor sodium hydrosulfide in diabetic nephropathy. Clin Exp Nephrol. 2013;17(1):32–40
- Renga B. Hydrogen sulfide generation in mammals: The molecular biology of cystathionine-beta-synthase (CBS) and cystathionine-gamma-lyase (CSE). Inflamm Allergy Drug Targets. 2011;10(2):85–91
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527–531
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016
- Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem. 2004;279(47):49199–49205
- Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H. Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci. 2002;22(9):3386–3391
- Kaneko Y, Kimura Y, Kimura H, Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: Possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–1397
- Calvert JW, Jha S, Gundewar S, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365–374
- Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia–reperfusion injury. Life Sci. 2008;82(23–24):1196–1202
- Kang K, Zhao M, Jiang H, et al. Role of hydrogen sulfide in hepatic ischemia–reperfusion-induced injury in rats. Liver Transpl. 2009;15(10):1306–1314
- Ersoy B. Hydrogen sulfide attenuates ischemia–reperfusion injury in in vitro and in vivo models of intestine free tissue transfer. Plast Reconstr Surg. 2011;127(1):487–488; author reply 488
- Tripatara P, Patel NS, Collino M, et al. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest. 2008;88(10):1038–1048
- Xu Z, Prathapasinghe G, Wu N, et al. Ischemia–reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. Am J Physiol Renal Physiol. 2009;297(1):F27–F35
- Tripatara P, Patel NS, Brancaleone V, et al. Characterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischemia/reperfusion injury of the mouse kidney: An in vivo study. Eur J Pharmacol. 2009;606(1–3):205–209
- Goncalves GM, Castoldi A, Braga TT, Camara NO. New roles for innate immune response in acute and chronic kidney injuries. Scand J Immunol. 2011;73(5):428–435
- Ben Mkaddem S, Pedruzzi E, Werts C, et al. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ. 2010;17(9):1474–1485
- Kim BS, Lim SW, Li C, et al. Ischemia–reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79(10):1370–1377
- Wolfs TG, Buurman WA, van Schadewijk A, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168(3):1286–1293
- Donnahoo KK, Meng X, Ayala A, et al. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia–reperfusion. Am J Physiol. 1999;277(3 Pt 2):R922–R929
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18(10):1165–1167
- Asimakopoulou A, Panopoulos P, Chasapis CT, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol. 2013;169(4):922–932
