Abstract
Objective: Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) reduce proteinuria in diabetic nephropathy (DN). Some studies have suggested that dual blockade of the renin–angiotensin system provides additive benefits in DN but others showed increased adverse events. We performed a meta-analysis to evaluate the efficacy and safety of combination therapy for DN. Methods: Studies were identified by searching MEDLINE, EMBASE, PubMed, and CNKI. All trials involved ACEI + ARB (combination therapy), and ACEI or ARB alone (monotherapy) for DN. The outcomes measured were urinary total proteinuria (UTP), urinary albumin excretion rate (UAER), serum creatinine, glomerular filtration rate (GFR), end-stage renal disease (ESRD), hyperkalemia, hypotension, and acute kidney injury (AKI). Results: In the 32 included trials, 2596 patients received combination therapy and 3947 received monotherapy. UTP and UAER were significantly reduced by combined treatment compared with monotherapy. It was notable that low doses of combination therapy reduced UTP more than high doses. Serum creatinine, GFR, and ESRD were not significantly different between the two groups. In severe DN, the occurrence of hyperkalemia and AKI were higher with combination therapy. However, in mild DN, the prevalence of hyperkalemia and AKI were the same in both the groups. In mild DN, the occurrence of hypotension was higher with combination therapy; however, in severe DN, it was not different between the two groups. Conclusion: Our meta-analysis suggests that combination therapy can be used on DN with proteinuria, but should be used with caution in those with decreased renal function, especially with severe renal failure.
Introduction
Diabetic nephropathy (DN) refers to the kidney disease caused by diabetes mellitus. DN is one of the most important microvascular complications in patients with diabetes, and is also the most common underlying cause of end-stage renal disease (ESRD).Citation1 The main indicators of DN are continuous albuminuria, high blood pressure, and progressive renal damage.Citation2 StudiesCitation3,Citation4 have shown that renin–angiotensin–aldosterone system (RAAS) blockers can control blood pressure, reduce proteinuria, inhibit renal fibrosis, and delay progression to DN. RAAS blockers mainly include direct renin inhibitor, angiotensin-converting enzyme inhibitors (ACEI), and angiotensin II receptor blocker (ARB). ACEI and ARB act at different stages in the RAAS: ACEI prevents conversion of Ang I to Ang II by inhibiting angiotensin-converting enzyme and ARB reduces the biological activity of Ang II by blocking the binding of Ang II to its type 1 receptor (AT1). It has been pointed outCitation5 that for patients with late DN, using ACEI or ARB alone is insufficient to completely preserve renal function over the long term. The Kidney Disease Outcomes Quality Initiative in 2012 concluded that patients with diabetes with normal blood pressure and with microalbuminuria (urinary albumin/creatinine 30–300 mg/g) can use ACEI or ARB drugs. One meta-analysisCitation6 examined combined ACEI and ARB therapy and found that it reduced 24-h proteinuria to a greater extent than therapy with ACEI alone for DN. Although these studies support the benefit of combination therapy, all the included articles were small. However, recently two large clinical trialsCitation7,Citation8 showed that combination therapy conferred no additional benefit in reducing progression to ESRD in individuals with patients with diabetes. At present, there are no clear guidelines concerning the optimal use of ACEI and ARB in patients with DN. The purpose of this meta-analysis is to pool the results of these studies in order to better understand the role of dual blockade of the RAAS in patients with DN.
Methods
Data sources and searches
We searched six electronic databases (MEDLINE, EMBASE, PubMed, and CNKI) between 1966 and January 2014 for randomized controlled clinical trials in which an ARB combined with an ACE inhibitor were used to treat patients who were diagnosed with DN, by using the following Medical Subject Headings (MeSH) and text words: angiotensin-receptor-blockers, ARB, angiotensin-converting enzyme inhibitor, ACEI, the generic names of currently available ARBs or ACEI (losartan, valsartan, irbesartan, candesartan, telmisartan, eprosartan, olmesartan, imidapril, enalapril, lisinopril, captopril, cilazapril, ramipril, perindopril, and fosinopril), proteinuria, albuminuria, microalbuminuria, and diabetic nephropathies. We searched for additional studies in the reference lists of all identified publications, including relevant meta-analyses and systematic reviews.
Study selection and quality assessment
Studies that met the following criteria were considered for inclusion in the study: randomized, controlled, parallel or crossover trials, which investigated patients with microalbuminuria or proteinuria of patients with diabetes and reported changes in urinary protein excretion during combination therapy with an ARB plus an ACE inhibitor versus monotherapy with either drug alone. We included control studies lasting at least 2 months with parallel-group or crossover designs. Studies were excluded for the following reasons: patients had undergone renal transplantation or had normal urinary protein excretion, or patients had chronic proteinuria due to non-diabetic causes. Studies that examined other combination therapies, or those that only compared various doses of the same drug, were also excluded.
The methodological quality of all included studies was rated according to Cochrane Collaboration’s tool Handbook 5.1 for the UKCitation9 which measures random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
Data extraction and statistical analysis
Titles, abstracts, and entire articles retrieved and regarded as potentially relevant by the third investigator were screened independently by the other two investigators. Two investigators abstracted data independently in duplicate on population (patient characteristics and sample size), methods (trial design), intervention (specific medications used, duration of treatment, and number of dropouts), and outcomes [urinary total proteinuria (UTP) mg/24 h] of urinary albumin excretion rate (UAER mg/24 h), serum creatinine (mmol/L), glomerular filtration rate (GFR) (ml/min), progression to ESRD, serum potassium (mmol/L), acute kidney injury (AKI), and hypotension]. Differences in inclusion of studies and/or interpretation of data were resolved by consensus or discussion with a third investigator.
Weighted mean differences and confidence interval for continuous variables and odds ratio and confidence interval for binary variables were calculated using Rev Man v. 5.2 (The Cochrane Collaboration, Oxford, UK) and utilizing a fixed-effects model. Statistical heterogeneity was assessed with a χ2-test. Statistical heterogeneity is present when significant variability was found in the results of the studies included in the meta-analysis. A p-value of <0.1 or an I2-value of >50% defined significant heterogeneity. Data of monotherapy groups containing any ACEI alone and of the ARB alone groups were included among our selected articles. We analyzed the heterogeneity resulting from the various medications and doses used by dividing the studies into subgroups with high doses (perindopril 8 mg, lisinopril 40 mg, enalapril 40 mg, captopril 150 mg + irbesartan 300 mg, candesartan 16 mg, and losartan 100 mg) and low doses (benazepril 10–20 mg, lisinopril 20 mg, enalapril 10–20 mg, ramipril 5 mg, perindopril 4 mg, temocapril 2 mg, captopril 100 mg + irbesartan 150 mg, valsartan 80 mg, candesartan 4–8 mg, and losartan 50 mg) of ACEI in combination with ARB. We divided the data into other subgroups according to the severity of the DN: individuals with DN whose estimated GFR was <60 ml/min/1.73 m2 of body-surface area and/or macroalbuminuria (albumin-to-creatinine ratio >1000 or protein-to-creatinine ratio >1.5) were grouped as patients with severe DN; those whose estimated GFR was >60 ml/min/1.73 m2; and/or normoalbuminuria, microalbuminuria (albumin-to-creatinine ratio ≤1000 or protein-to-creatinine ratio ≤1.5) were grouped as patients with mild DN. Those whose serum creatinine >0.3 mg/dl or increased 50% than the previous levels; and/or urine decreased to <0.5 ml/kg h which persist >6 h were grouped as patients with AKI. Hypotension (blood pressure is <90/60 mmHg) referred to the excessive drops in blood pressure due to treatment.
Results
Trial flow and study characteristics and quality
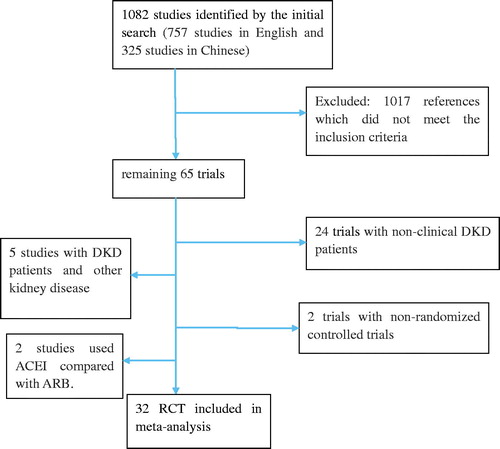
Our initial search identified 1082 studies (757 studies in English and 325 studies in Chinese). All the Chinese trials were selected from CSSCI. shows the search results and selection of the randomized trials for analysis. By reading the title, abstract, and the full text, we preliminarily ruled out 1017 references which did not meet the inclusion criteria, after which 65 trials remained. We reviewed the remaining full articles further and excluded 24 trials which included individuals with non-clinical DN, 5 studiesCitation10–14 which included patients with DN as well as other kidney disease, 2 trialsCitation15,Citation16 which were not correctly controlled, and 2 studiesCitation5,Citation17 in which ACEI was compared with ARB. The final meta-analysis included 32Citation7,Citation8,Citation18–47 trials (22 studies in English and 10 in Chinese) (), 10 of which were crossover design while the remainders were parallel group design. The characteristics of the included studies are listed in . In the 32 included trials (n = 6543), 2596 patients received ACEI + ARB and 3947 received ACEI or ARB alone.
Table 1. Comparison of trials with combination RAAS therapy for diabetic nephropathy.
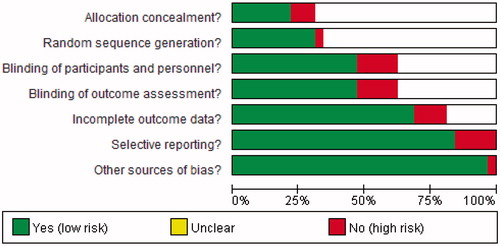
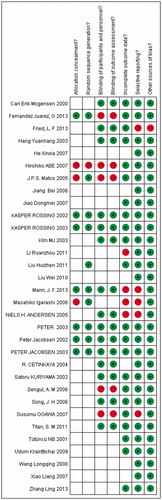
The quality of the 32 studies included was as follows ( and ): 5 studies met all 7 quality criteria [concealed allocation, blinding (participants and personnel, outcome assessment), random, incomplete outcome data, selective reporting, and other sources of bias], whereas 8 studies met 5 of the criteria, 3 studies met 4 criteria, 8 studies met 3 criteria, and the remaining 8 studies met 2 of the criteria. Only 7 articles mentioned allocation concealment, 10 articles mentioned random sequence generation, 16 studies were described as double-blind suggesting low risk of selection bias, and 22 articles reported incomplete outcome data suggesting high risk of bias.
Quantitative data analysis
Efficacy end point
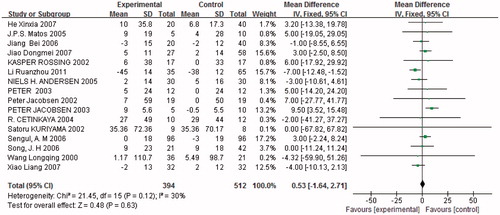
Urinary total proteinuria
Fifteen studies (n = 649) demonstrated a difference in 24 h proteinuria between combined treatment and monotherapy. Proteinuria was significantly reduced in trials with combined treatment [p < 0.05, weighted mean differences (MD) = −178.97, 95% confidence intervals (95% CI) (−203.47, −154.46), ]. Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 0%, p = 0.5).
Figure 4. Figures 4a–12c present the results of the meta-analysis. The black diamond represents the weighted mean difference (WMD) or odds ratio (OR) and 95% confidence interval. Statistical heterogeneity is demonstrated by p = 0.07–0.91 and I2 = 0–70%. Statistical significance of differences is demonstrated by p < 0.05 or p > 0.05. Experimental = combination therapy (ACEI + ARB); control = monotherapy (ACEI or ARB). (A) Combination therapy versus monotherapy, outcome: UTP (mg/24 h); p < 0.05; p = 0.50; and I2 = 0%. (B) Low-dose combination therapy versus monotherapy, outcome: UTP (mg/24 h); p < 0.05; p = 0.28; and I2 = 19%. (C) High-dose combination therapy versus monotherapy, outcome: UTP (mg/24 h); p = 0.07; p = 0.67; and I2 = 0%.
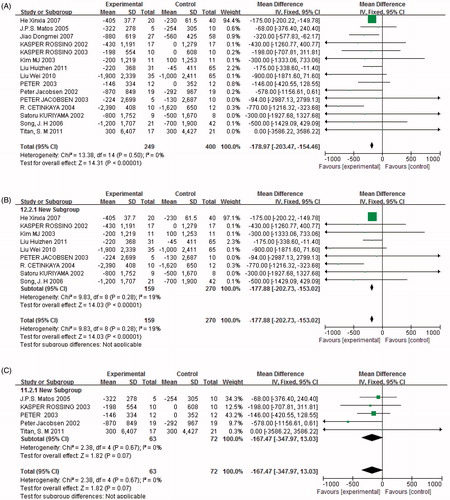
The subgroups were divided into high and low doses of ACEI and ARB combination treatment. Proteinuria was significantly reduced in trials with low doses of combined treatment [p < 0.05, MD = −177.88, 95% CI (−202.73, −153.02), ], but there was no significant difference between high doses of combined treatment and monotherapy [p = 0.07, MD = −167.47, 95% CI (−347.97, 13.03), ]. The results revealed that low doses of ACEI in combination with ARB had a greater effect in reducing proteinuria than high doses.
Urinary albumin excretion rate
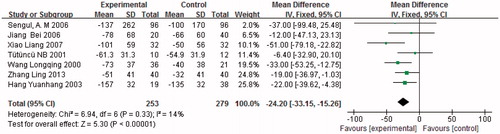
Seven studies (n = 532) showed a reduction in UAER. UAER was significantly reduced in combined treatment trials compared with monotherapy [p < 0.05, MD = −24.20, 95% CI (−33.15, −15.26), ]. Heterogeneity testing again showed no statistically significant difference between these studies (I2 = 14%, p = 0.33). All patients treated for an elevated UAER received low doses of ACEI in combination with ARB. Consequently, we did not divide the studies into subgroups according to the therapeutic dose used to treat UAER.
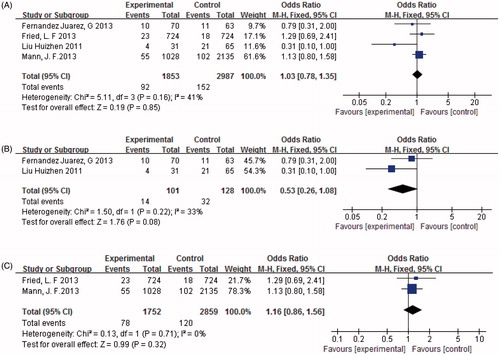
End-stage renal disease
Four trials (n = 4840) reported progression of ESRD. There was no evidence of an additional benefit of combined therapy in reducing progression to ESRD (p = 0.85, ). Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 41%, p = 0.16). The subjects were then divided into subgroups of severe and mild DN with combination therapy. The findings revealed that there was no significant difference in either mild or severe DN treated with combination therapy or monotherapy in reducing progression to ESRD (p = 0.32 and p = 0.08, .
Figure 6. (A) Combination therapy versus monotherapy, outcome: ESRD; p = 0.85; p = 0.16; and I2 = 41%. (B) Severe diabetic nephropathy with combination therapy versus monotherapy, outcome: ESRD; p = 0.08; p = 0.22; and I2 = 33%. (C) Mild diabetic nephropathy with combination therapy versus monotherapy, outcome: ESRD; p = 0.32; p = 0.71; and I2 = 0%.
Serum creatinine
Sixteen studies (n = 906) showed a change in serum creatinine, with no significant difference between the two groups (p = 0.63, ). All the patients examined for serum creatinine had mild DN, so we did not divide the subjects into subgroups for serum creatinine.
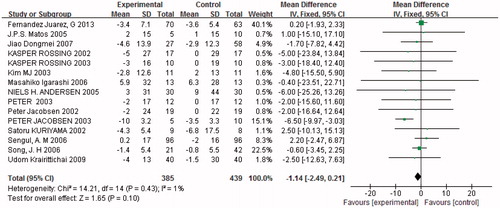
Glomerular filtration rate
Fifteen trials (n = 824) showed alterations in GFR, but there was no significant difference between the two groups (p = 0.1, ). Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 1%, p = 0.43). All the patients evaluated for GFR had mild DN, so we did not divide the subjects into subgroups for GFR.
Safety end points
Potassium
Two subgroups were established according to the nature of the observation indices, depending on whether data were continuous variables (serum potassium) or dichotomous variables (hyperkalemia determined by the number of patients whose potassium was >5.0 mmol/L). Fourteen trials (n = 717) showed an alteration in serum potassium. Levels were significantly increased with combined treatment compared with monotherapy [p < 0.05, MD = 0.17, 95% CI (0.09, 0.24), ]. Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 22%, p = 0.22). Eighteen studies (n = 5529) provided data on adverse events associated with hyperkalemia, which developed significantly more frequently with combined treatment than with monotherapy [p < 0.05, odds ratio (OR) = 1.79, 95% CI (1.46, 2.19), ]. Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 17%, p = 0.28). We established subgroups for hyperkalemia according to the severity of DN. Our findings revealed that hyperkalemia occurred more frequently in patients with severe DN treated with combination therapy than in those treated with monotherapy [p < 0.05, OR = 1.87, 95% CI (1.51, 2.32), ]. In contrast, there was no significant difference in the incidence of hyperkalemia between combination therapy and monotherapy in patients with mild DN (p = 0.29, ). All the patients examined for serum potassium were diagnosed with mild DN, so we did not divide the patients into subgroups for serum potassium.
Figure 9. Combination therapy versus monotherapy, outcome: Serum potassium (mmol/L); p < 0.05; p = 0.22; and I2 = 22%.
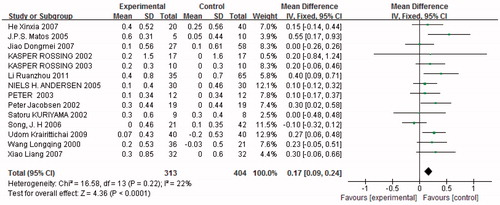
Figure 10. (A) Combination therapy versus monotherapy, outcome: hyperkalemia; p < 0.05; p = 0.28; and I2 = 17%. (B) Severe diabetic nephropathy with combination therapy versus monotherapy, outcome: hyperkalemia; p < 0.05; p = 0.07; and I2 = 70%. (C) Mild diabetic nephropathy with combination therapy versus monotherapy, outcome: hyperkalemia; p = 0.29; p = 0.52; and I2 = 0%.
Hypotension
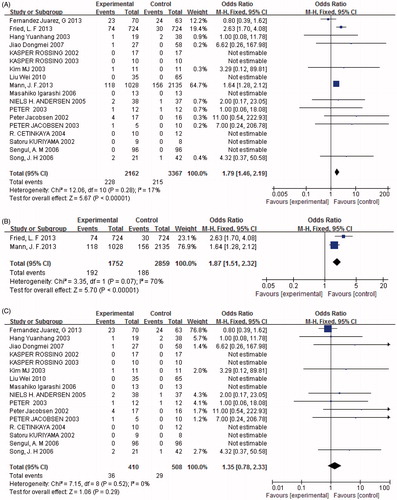
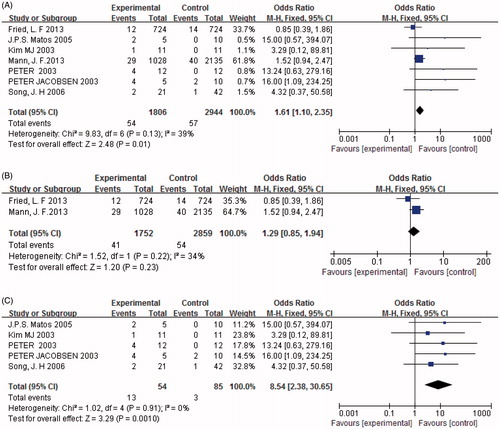
Seven studies (n = 4750) presented data on the occurrence of hypotension, and found a significant difference between combined treatment and monotherapy. The number of individuals with hypotension in the combined treatment group was significantly greater than the monotherapy group [p < 0.05, OR = 1.61, 95% CI (1.10, 2.35), ]. Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 39%, p = 0.13). Furthermore, when we established subgroups for hypotension according to the severity of DN. The results revealed that hypotension developed more frequently in those with mild DN treated with combination therapy rather than monotherapy [p < 0.05, OR = 8.54, 95% CI (2.38, 30.65), ]. In contrast, there was no significant difference in the incidence of hypotension in individuals with severe DN treated with combination therapy compared with monotherapy (p = 0.23, ).
Figure 11. (A) Combination therapy versus monotherapy, outcome: hypotension; p < 0.05; p = 0.13; and I2 = 39%. (B) Severe diabetic nephropathy with combination therapy versus monotherapy, outcome: hypotension; p = 0.23; p = 0.22; and I2 = 34%. (C) Mild diabetic nephropathy with combination therapy versus monotherapy, outcome: hypotension; p < 0.05; p = 0.91; and I2 = 0%.
Acute kidney injury
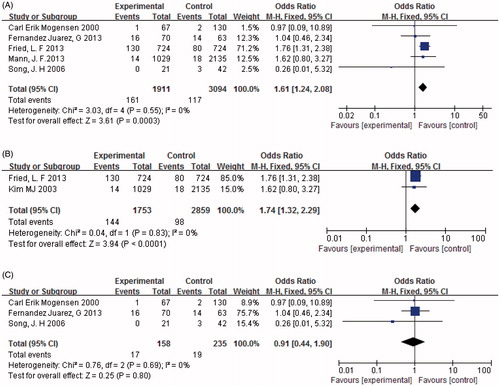
Five papers (n = 5055) provided data on AKI as an adverse event. It was found to occur significantly more frequently in those treated with combined therapy compared to monotherapy [p < 0.05, OR = 1.61, 95% CI (1.24, 2.08), ]. Heterogeneity testing showed that there was no statistically significant difference between the studies (I2 = 0%, p = 0.55). When patients were divided into subgroups according to the severity of DN, the results revealed that AKI occurred more frequently in those with severe DN treated with combination therapy than with monotherapy [p < 0.05, OR = 1.74, 95% CI (1.32, 2.29), ]. In contrast, there was no significant difference between individuals with mild DN treated with combination therapy and those receiving monotherapy (p = 0.8, ) in the occurrence of AKI.
Figure 12. (A) Combination therapy versus monotherapy, outcome: AKI; p < 0.05; p = 0.55; and I2 = 0%. (B) Severe diabetic nephropathy with combination therapy versus monotherapy, outcome: AKI; p < 0.05; p = 0.83; and I2 = 0%. (C) Mild diabetic nephropathy with combination therapy versus monotherapy, outcome: AKI; p = 0.80; p = 0.69; and I2 = 0%.
Discussion
DN is one of the most common complications of patients with diabetes, and is a significant cause of disability and death in patients with diabetes. The main pathological changes of DN are thickening and increased permeability of the glomerular basement membrane, increased proliferation of mesangial cells, and increased extracellular matrix, leading to glomerulosclerosis. When progressing to the clinical stage of DN, symptoms of massive proteinuria, hypertension, and damage to renal function often also develop. The available treatments for DN involve controlling blood pressure, blood fat and glucose, resisting oxidative stress, improving microcirculation, protecting kidney function, and conducting other comprehensive treatment measures, but in spite of such measures, the disease often progresses to end-stage renal failure in 7–10 years.
ACEI and ARB are widely used in the treatment of DN. The pathophysiology for dual blockade of the RAAS is based on the multiple pathways which inhibit angiotensin II and aldosterone. Blockade of the RAAS can diminish renal perfusion and delay proliferation of mesangial cells, as well as increased extracellular matrix production and glomerulosclerosis. Monotherapy with ACEI or ARB provides an incomplete blockade of the RAAS, since in the absence of ACE, other enzymes such as chymase can lead to the generation of angiotensin II.Citation48,Citation49 ACEI prolongs the half-life of bradykinin, while ARB does not, and bradykinin is a potent vasodilator which is believed to be renoprotective.Citation50 Thus, combination therapy could be expected to be more effective than monotherapy in opposing the RAAS. UTP, UAER, serum creatinine, GFR, and ESRD are all important indicators which determine the curative effect. However, it has been reported that combination therapy increases hyperkalemia and hypotension, reduces renal function, and is associated with AKI.
Our meta-analysis revealed that, compared with monotherapy, 24 h proteinuria and UAER were significantly lowered in patients with clinical DN receiving combination therapy. The medications and doses employed in the studies were inconsistent and this may introduce clinical heterogeneity (). Some included studies used both low and high doses of ACEI and ARB in combination; some included studies used addition of an ARB to submaximal doses of an ACEI. Furthermore, the optimal dose for ACEI and ARB to combat proteinuria has never been determined. The clinical heterogeneity was evaluated by analyzing subgroups divided according to high and low doses of ACEI and ARB in combination treatment. We divided all the trials into a low- and a high-dose group. However, the subgroup analysis revealed that low doses of ACEI and ARB in combination reduced proteinuria more than high doses. The studies included in this analysis measured proteinuria only over the short-term (most studies were 8–20 weeks in duration), so the data of this meta-analysis was only able to provide insight into the effect of recent dual RAAS blockade. The ONTARGETCitation8 and VA NEPHRON-DCitation7 studies (the two studies that were at least 2 years in duration) suggested that combination therapy is superior to monotherapy in reducing 24-h urinary protein (Here, we only discuss the function of reducing urinary protein except for the adverse reactions that will be discussed in the following paragraphs.); therefore, this suggests that combination therapy can reduce proteinuria better over longer durations. These two reports are not included in the analysis of proteinuria in this study because they did not provide the standard deviation. The baseline level of protein excretion (patients with macroalbuminuria or nephrotic range proteinuria were included) and the stage of DN (patients with DN stages 2–4 were included) of those articles included in our study were variable. This may introduce clinical heterogeneity since we evaluated effectiveness by determining percent reduction in protein excretion. In conclusion, our meta-analysis suggests that low doses of ACEI and ARB in combination can reduce proteinuria in cases of patients with clinical diabetes.
There was no significant difference between the two groups with regard to serum creatinine, GFR or ESRD (either in overall analysis or in subgroup analysis). Four trials (n = 4840) reported progression of ESRD. Study 7 included 724 patients with DN and study 8 included 1028 patients with DN treated with combination therapy whose estimated GFR was <60 ml/min/1.73 m2 of body-surface area and/or macroalbuminuria. The conclusion may be associated with the fact that most patients in whom ESRD was observed had severe DN. Moreover, fewer (n = 101) patients with mild DN were treated with combination therapy and this may affect the results. Therefore, in future researchers should develop a trial to confirm whether combination therapy can delay progression of mild DN to ESRD.
The occurrence of AKI and hyperkalemia were higher in the combination therapy group than in the monotherapy group of patients with severe DN. The subgroups for AKI revealed that AKI occurred more frequently in those with severe DN treated with combination therapy than with monotherapy while there was no significant difference in the occurrence of AKI between combination therapy and monotherapy in those with mild DN. This phenomenon may be related to the fact that patients with severe DN are more sensitive to the reduced renal perfusion than those with mild DN. The subgroups of the dichotomous variable hyperkalemia produced the same conclusion as AKI analysis. In individuals with severe DN, hyperkalemia occurred more frequently with combination therapy than monotherapy, but in those with mild DN, the incidence of hyperkalemia was similar in the two groups. The continuous variable serum potassium increased with combined treatment compared with monotherapy. The patients who were involved in the study of serum potassium were all diagnosed with mild DN. The conclusion was not consistent with the conclusion of subgroup analysis of hyperkalemia in patients with mild DN. This may be due to the fact that the definition of the two outcomes was different. The serum potassium index (continuous variable) revealed an increase in potassium, but the hyperkalemia index (dichotomous variable) evaluated the number of patients whose potassium was >5.0 mmol/L. In conclusion, the results suggest that we should pay attention to the adverse events of AKI and hyperkalemia in patients with severe DN receiving combined treatment. The occurrence of hypotension was higher in those individuals with mild DN treated with combination therapy compared to monotherapy, but there was no significant difference between combination therapy and monotherapy in those with severe DN. This result may be related to the fact that blood pressure is more prone to rise in severe DN than mild DN, so the possibility of hypotension is low in patients with severe DN receiving combination therapy. Consequently, we should watch for hypotension in patients with mild DN receiving combination therapy.
This meta-analysis included 32 articles, among which only seven mentioned allocation concealment, 10 mentioned random sequence generation, only 16 studies mentioned double-blinding, and 3 were multicenter studies. The quality of these articles was variable and that adds error and bias to the meta-analysis. Some studies absorbed in our analysis did not report in detail adverse reactions encountered with combination therapy and some did not accurately report the number of adverse reactions in the trial group and control group respectively, leading to some adverse reaction indices which could not be accurately statistically analyzed. In future researchers should perform higher quality, multicenter, randomized, double-blind controlled trials to confirm the curative effect of ACEI and ARB combination therapy in patients with clinical DN to guide clinical therapy.
In conclusion, according to the results of our meta-analysis: in DN, the combination of ACEI and ARB is able to reduce 24 h proteinuria and UAER better than either ACEI or ARB alone. The combination of low doses of ACEI and ARB is better in reducing proteinuria, but there is no additional benefit in relieving ESRD in cases of serious DN. Moreover, combination therapy increases the incidence of adverse reactions (such as hyperkalemia and AKI), especially for those with serious DN. The occurrence of hypotension is also higher in those with mild DN receiving combination therapy. To summarize, our meta-analysis suggests that low doses of ACEI and ARB in combination can be used to treat patients with diabetes and proteinuria, but should be used with caution on patients with diabetes with reduced renal function, especially those with severe renal failure. The randomized controlled trials (RCTs) included in the present analysis gave priority to those with severe DN, or the follow-up time of the RCTs was shorter; in future, researchers should develop higher quality, multicenter, randomized, double-blind controlled trials to confirm the curative effect of ACEI and ARB combination therapy in patients with clinical DN, and in particular, provide data on long-term follow-up of individuals with mild DN to guide clinical therapy.
Acknowledgments
We would like to express our gratitude to those who provided their time and assistance for this study.
Declaration of interest
This study was supported by grant 81200519 from the National Natural Science Foundation of China.
References
- Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005;94:S14–S18
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. Diabetic Nephropathy. Diabetes Care. 2003;26(Suppl 1):S94–S98
- Eurich DT, Majumdar SR, Tsuyuki RT, Johnson JA. Reduced mortality associated with the use of ACE inhibitors in patients with type 2 diabetes. Diabetes Care. 2004;27:1330–1334
- Imai E, Chan JC, Ito S, et al. Effects of olmesartan on renal and cardiovascular outcomes in Type 2 diabetes with overt nephropathy: A multicentre, randomized, placebo-controlled study. Diabetologia. 2011;54:2978–2986
- Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961
- Jennings DL, Kalus JS, Coleman CI, Manierski C, Yee J. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: A meta-analysis. Diabet Med. 2007;24:486–493
- Linda FF, Nicholas E, Jane HZ, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. New Engl J Med. 2013;369:1892–1903
- Mann JF, Anderson C, Gao P, et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: Results of the ONTARGET Trial. J Hypertens. 2013;31:414–421
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed October 1, 2011
- Agarwal A. Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney Int. 2001;59:2282–2289
- Campbell R, Sangalli F, Perticucci E, et al. Effects of combined ace inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003;63:1094–1103
- Mann JF, Schmieder RE, ONTARGET Investigators, et al. Renal outcomes with telmisartan, ramipril, or both in people at high vascular risk (the Ontarget Study): A multicentre, randomized, double-blind, controlled trial. Lancet. 2008;372:547–553
- Segura J, Praga M, Campo C, Rodicio JL, Ruilope LM. Combination is better than monotherapy with ace inhibitor or angiotensin receptor antagonist at recommended doses. J Renin Angiotensin Aldosterone Syst. 2003;4:43–47
- Vidt DG. Telmisartan, ramipril, or both in patients at high risk for vascular events. Curr Hypertens Rep. 2008;10:343–344
- Fujisawa T, Ikegami H, Ono M, et al. Combination of half doses of angiotensin type 1 receptor antagonist and angiotensin-converting enzyme inhibitor in diabetic nephropathy. Am J Hypertens. 2005;18:13–17
- Hebert LA, Falkenhain M, Nohman N, Cosio FG, O’Dorisio TM. Combination ACE inhibitor and angiotensin II receptor antagonist in diabetic nephropathy. Am J Nephrol. 1999;19:1–6
- Barnett A. Prevention of loss of renal function over time in patients with diabetic nephropathy. Am J Med. 2006;119:S40–S47
- Hirohiko ABE, Shinya M, Hiroshige O, et al. Renoprotective effect of the addition of losartan to ongoing treatment with an angiotensin converting enzyme inhibitor in type-2 diabetic patients with nephropathy. Hypertens Res. 2007;30:929–935
- Andersen NH, Poulsen PL, Knudsen ST, Poulsen SH, Eiskjaer H, Hansen KW. Long-term dual blockade with candesartan and Lisinopril in hypertensive patients with diabetes. Diabetes Care. 2005;28:273–277
- Cetinkaya R, Odabas AR, Selcuk Y. Anti-proteinuric effects of combination therapy with enalapril and losartan in patients with nephropathy due to type II diabetes. Int J Clin Pract. 2004;58:432–435
- Fernandez Juarez G, Luno J, Barrio V, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: A randomized trial. Am J Kidney Dis. 2013;61:211–218
- Igarashi M, Hirata A, Kadomoto Y, Tominaga M. Dual blockade of angiotensin II with enalapril and losartan reduces proteinuria in hypertensive patients with type 2 diabetes. Endocr J. 2006;53:493–501
- Jacobsen P, Anderson S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in Type 1 diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14:992–999
- Jacobsen P, Anderson S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin–angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63:1874–1880
- Jacobsen P, Andersen S, Rossing K, Hansen BV, Parving HH. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol Dial Transplant. 2002;17:1019–1024
- Kim MJ, Song JH, Suh JH, Lee SW, Kim GA. Additive anti-proteinuric effect of combination therapy with ACE inhibitor and angiotensin receptor antagonist: Differential short-term response between IgA nephropathy and diabetic nephropathy. Yonsei Med J. 2003;44:463–472
- Krairittichai U, Chaisuvannarat V. Effects of dual blockade of renin-angiotensin system in type 2 diabetes mellitus patients with diabetic nephropathy. J Med Assoc Thai. 2009;92:611–617
- Kuriyama S, Tomonari H, Tokudome G, et al. Anti-proteinuric effects of combined antihypertensive therapies in patients with overt type 2 diabetic nephropathy. Hypertens Res. 2002;25:849–855
- Matos JPS, de Lourdes Rodrigues M, Ismerim VL, Boasquevisque EM, Genelhu V, Francischetti EA. Effects of dual blockade of the renin angiotensin system in hypertensive type 2 diabetic patients with nephropathy. Clin Nephrol. 2005;64:180–189
- Mogensen CE, Neldam S, for the CALM study group, et al. Randomized controlled trial of the dual blockade of renin–angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: The candesartan and Lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–1444
- Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens Res. 2007;30:325–334
- Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: A randomized double-blind crossover trial. Diabetes Care. 2003;26:2268–2274
- Rossing K, Christensen PK, Jensen BR, Parving HH. Dual blockade of the renin–angiotensin system in diabetic nephropathy: A randomized double-blind crossover study. Diabetes Care. 2002;25:95–100
- Sengul AM, Altuntas Y, Kurklu A, Aydin L. Beneficial effect of lisinopril plus telmisartan in patients with type 2 diabetes, microalbuminuria and hypertension. Diabetes Res Clin Pr. 2006;71:210–219
- Song JH, Cha SH, Lee HJ, et al. Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant. 2006;21:683–689
- Tütüncü NB, Gurlek A, Gedik O. Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: A prospective study. Acta Diabetol. 2001;38:157–161
- Titan SM, Vieira JM Jr, Dominguez WV, Barros RT, Zatz R. ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: A double-blind randomized clinical trial. Clin Nephrol. 2011;76:273–283
- Bei J, Xianhua L, Xiangdong Y, Jian S, Ling G, Zhao H. Renoprotective effects of dual blockade of renin-angiotensin system in diabetic nephropathy. J Shandong Univ (Health Sci). 2006;59:896–899
- Xinxia H, Weihong H, Yan X, Hongyan W, Le W. Clinical study of the protective effect of ACEI and ARB on patients with diabetic nephropathy. Chin J Prev Control Chronic Dis. 2007;15:34–35
- Yuan-Hang H, Haitao W, Qizhi Z, Hong Z, Wen S, Yin W. Combination therapy with losartan and fosinopril for early diabetic nephropathy. J First Mil Med Univ. 2003;23:963–965
- Dongmei J, Wei L, Shangwen W, Zhenzhong X. The efficiency of combined treatment with benazepril and valsartan on thirty patients with diabetic kidney disease. Shandong Med J. 2007;47:69–70
- Yuan-Zhou L, Guiping L, Aiwen C, Ruyi Z. The efficacy of combined treatment with benazepril and losartan on early type 2 diabetic nephropathy and hypertension. Guangdong Med J. 2011;32:2991–2993
- Huizhen L, Wen L, Jinrong L, Qiujun Z, Xiuyun L, Gaiying H. The efficiency of dual blockade of the renin-angiotensin system on patients with diabetic nephropathy. Chin J Gerontol. 2011;31:1964–1966
- Wei L. The efficiency of combined treatment with perindopril and valsartan on diabetic nephropathy. Shandong Med J. 2010;50:107–108
- Longqing W, Lixia L, Junyan M. Clinical application of combination ACEI with losartan in treating diabetic nephropathy. J Beihua Univ (Nat Sci). 2000;1:506–508
- Liang X, Shuhong Z. The efficiency of combined treatment with imidapril and valsartan on thirty-two patients with early diabetic nephropathy. China J Mod Med. 2007;17:1130–1131
- Ling Z, Jiansheng N. The efficacy and safety of combined treatment with enalapril and losartan on early type 2 diabetic nephropathy and hypertension. Chin J Gerontol. 2013;33:2038–2040
- Hollenberg NK, Fisher NDL, Price DA. Pathways for angiotensin II generation in intact human tissue: Evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–392
- Mckelvie RS, Yusuf S, Pericak D, Gomis R, Andersen S, Arner P. Comparison of candesartan, enalapril, and their combination in congestive heart failure. The RESOLVD Pilot Study Investigators. Circulation. 1999;100:1056–1064
- Imig JD. ACE inhibition and bradykinin-mediated renal vascular responses: EDHF involvement. Hypertension. 2004;43:533–535