Abstract
Chronic kidney disease (CKD) is an important global health problem that affects 8–15% of the population according to epidemiological studies done in different countries. Essential to prevention is the knowledge of the environmental factors associated with this disease, and heavy metals such as lead and cadmium are clearly associated with kidney injury and CKD progression. Arsenic is one of the most abundant contaminants in water and soil, and many epidemiological studies have found an association between arsenic and type 2 diabetes mellitus, hypertension and cancer; however, there is a scarcity of epidemiological studies about its association with kidney disease, and the evidence linking urinary arsenic excretion with CKD, higher urinary excretion of low molecular proteins, albuminuria or other markers of renal in injury is still limited, and more studies are necessary to characterize the role of arsenic on renal injury and CKD progression. Global efforts to reduce arsenic exposure remain important and research is also needed to determine whether specific therapies are beneficial in susceptible populations.
Introduction
Chronic kidney disease (CKD) is an important global health problem that affects 8–15% of the population according to epidemiological studies done in different countries, and is essential to prevent to recognize the environmental factors associated with this disease.Citation1
Some forms of CKD have been associated with environmental nephrotoxins such as heavy metals exposure,Citation2,Citation3 mycotoxins produced by fungi in improperly stored foods,Citation4 air pollutantsCitation5 and pesticides.Citation6 Examples of new classes of CKD linked with environmental factors are the BalkansCitation7 and Mesoamerican nephropathy.Citation8 Cadmium and lead exposure are of particular concern as many epidemiological studies have shown a strong association between exposure to this heavy metals, markers of renal injury and CKD progression.Citation9
Arsenic exposure remains a major public health problem as millions of people are exposed to water levels above the limit. As is present in nature in inorganic (arsenite, arsenate) or organic compounds (arsenobetaine, arsenocholine and arsenosugars); arsenobetaine is the most frequent compound in fish and is non-toxic to humans, whereas arsenite is mostly found in drinking water and is highly toxic to humans.Citation10
Arsenic is associated with formation of tumors in skin, lungs, bladder, liver and kidneys;Citation11,Citation12 arsenic exposure has also been recognized as a risk factor for cardiovascular disease,Citation13 hypertension,Citation14 peripheral artery diseaseCitation15 and diabetes mellitus;Citation16 nevertheless the recognition of arsenic exposure as a risk factor for renal disease is recent, and the number of studies and epidemiological data are still scarce. To evaluate the role of arsenic in kidney disease we conducted an analysis looking for studies about the physiopathology and epidemiology of arsenic exposure and nephrotoxicity.
We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com/home) and TOXLINE (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgenTOXLINE) databases to find all published studies evaluating the relationship between arsenic exposure with renal or kidney disease using the free text and Medical Subject Headings (MeSH) terms “arsenic”, “arsenate” or “arsenite” and “kidney”, “chronic kidney disease”, “tubular dysfunction” or “proteinuria”. The search period was from January 1965 through March 2014 with no language restrictions.
Early biomarkers to evaluate arsenic exposure and renal injury
The United States National Institutes of Health has defined the term “biomarker” as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention”. Biomarkers are by definition objective and quantifiable characteristics of biological processes, and could only serve as true replacements for clinical relevant end-points if we understood the normal physiology of a biological process, the pathophysiology of that process in the disease state and the effects of an intervention on these processes.Citation17
One of the major challenges in the fields of As toxicology has involved the monitoring of risk populations for early signs of exposure and toxicity. A useful biomarker of arsenic nephrotoxicity has to be associated with a statistically significant increase in the relative risk (odds ratio or hazard ratio) of developing progressive renal disease, CKD and/or an increase in the morbi-mortality related to renal injury.Citation18
Epidemiological and experimental studies evaluating arsenic nephrotoxicity had used the measurement of the glomerular filtration rate (GFR), proteinuria, albuminuria, N-acetyl-β-d-glucosaminidase (NAG), β2-microglobulin, α1-microglobulin and retinol-binding protein as markers of renal toxicity ().
Table 1. Summary of different outcomes related with arsenic exposure and renal disease.
Estimates of GFR are the best tools to evaluate the level of kidney function, and equations that take into account the serum creatinine and some variables such as age, gender, race and body size provide useful estimates of GFR; serum creatinine alone is not an accurate index to evaluate the GFR.Citation19
Normal individuals usually excrete very small amounts of protein in the urine and persistently increased protein excretion is usually a marker of kidney damage. The excretion of specific types of protein, such as albumin and/or low molecular weight proteins, depends on the type of kidney disease that is present. Increased excretion of albumin is a sensitive marker for kidney disease due to diabetes, glomerular disease and hypertension, whereas an increased excretion of low molecular weight proteins is a sensitive marker for some types of tubule-interstitial disease.Citation20
Microalbuminuria is defined as the urinary excretion of 30–300 mg/d in a timed urine collection in adults, and macroalbuminuria as a urinary excretion higher than 300 mg/d. Albuminuria has long been recognized as a marker for renal disease and is a well-known predictor of poor renal and cardiovascular outcomes in patients with type 2 diabetes and essential hypertension;Citation21 albuminuria indicates glomerular and endothelial injury,Citation22 and some studies had used this marker as clinical subrogate of kidney disease related to arsenic exposure,Citation23 the addition of albumin measurement to estimated GFR best classifies the population at risk for renal impairment;Citation24 however, the significance of isolated microalbuminuria as a risk factor for CKD progression is still controversial.Citation25
Urinary enzymes or low molecular-weight (LMW) plasma proteins that are normally freely filtered through the glomerulus have been used as specific markers of tubular injury. The impaired reabsorption of these proteins through the proximal tubule results in an increased excretion in urine and low-molecular weight proteinuria. Proteins with a molecular weight lower than 40 kDa, such as β2-microglobulin, α1-microglobulin and retinol-binding protein, are freely filtered through the glomerular membrane, and when tubular dysfunction is present their reabsorption in this segment is reduced.Citation26–28
NAG is a high molecular-weight lysosomal enzyme found in many tissues of the body, is not filtered through the glomerular membrane due to its high molecular weight. This enzyme is highly expressed in renal proximal tubular cells, and leaks into the tubular fluid when proximal tubular cells are injured as in arsenic exposure, its urine level increases and thus is used as a reflection of proximal tubular cell necrosis, however the role of this protein as marker of risk for progression of renal disease is still unknown.Citation29
Other urinary markers such as KIM-1 (Kidney Injury Molecule-1), NGAL (neutrophil gelatinase-associated lipocalin) or interleukin-18 have not been used to evaluate arsenic nephrotoxicity.
Metabolism and pathophysiology of arsenic-mediated nephrotoxicity
Inorganic arsenic ingested in drinking water is efficiently (80–90%) absorbed by the intestine, and some of the proteins that have been characterized as arsenic intestinal transporters are the aquaporin-10, the GLUT-5 and the organic anion transporting polypeptides (OATPB);Citation30 nutritional deficiencies and low intake of selenium, folic acid and vitamin B are associated with higher arsenic absorption and toxicity.Citation31,Citation32 Other routes of entry into the body are by inhalation and dermal exposure.Citation33
There are noticeable differences in the half-life of arsenic in blood between animal species, and in humans most arsenic can be rapidly eliminated from blood with a half-life of about 1 h.
In the liver, arsenite is taken up by the hepatocytes in a mechanisms mediated by aqua-glyceroporins (AQ) and hexose-permeases, whereas the arsenate goes into the cell by a mechanism mediated by the Na–P co-transporter.Citation34,Citation35 Studies done in cell cultures have shown that an increase in the cellular expression of AQ3 and AQ9 increases the intracellular accumulation of As.Citation36
In the cell, arsenate is transformed in arsenite and is methylated in a glutathione (GSH)-mediated process which decreases its toxicity and facilitates its biliary and urinary excretion.Citation37
Arsenic induces synthesis of a group of proteins called metallothioneins (MT) that are cysteine-rich, metal-binding proteins expressed in all eukaryotes and some prokaryotes cells. These proteins are highly inducible by heavy metals, and represent a major mechanism of metal detoxification in the body to maintain trace elements within a physiological range thus protecting the body from the damage by metal overload. Metallothioneins regulate metal homeostasis by sequestering metals in protein-bound forms, and serving as a scavenger to quench reactive oxygen species (ROS) and other free radicals.Citation38 MT knockout (MT-null) mice are more susceptible to nephrotoxicity induced by chronic exposure to heavy metals.Citation39
The ATP-binding cassette transporter proteins, multidrug resistance protein 1 (MRP1/ABCC1) and the related protein MRP2 (ABCC2) play an important role in arsenic detoxification through the cellular efflux of arsenic–GSH conjugates to the bile. The MRP-2 transporter is also located in proximal tubule cells (PTCs) to mediate the efflux of As to be excreted by urine.Citation40
Arsenic toxicity in PTC () is initiated by depletion of the intracellular GSH stores, activation of the caspase-3 and -9 signaling pathway, increases in the expression of interleukin-6 and interleukin-8 and activation of the p-53 apoptotic pathway. This leads to an increment in the production of ROS and other free radicals, inflammation and apoptosis.Citation41–43
Figure 1. Pathophysiology of arsenic-mediated nephrotoxicity. Mechanisms whereby arsenic exposure may lead to proximal tubular injury.
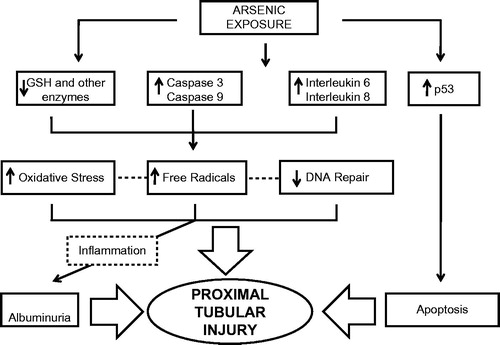
Arsenic uncouple oxidative phosphorylation causing reductions in sodium, phosphate and glucose transport, which is manifested clinically as Fanconi syndrome (phosphaturia, glucosuria and low-molecular weight proteinuria).Citation44
Albuminuria associated with As nephrotoxicity may be related to direct podocyte injury,Citation45 or through a mechanism related with endothelial dysfunction as has been shown that As increase the expression of the vascular cell adhesion molecule 1 (VCAM-1) and the angiotensin type I receptor.Citation46
In dogs, low-dose administration of sodium arsenate (0.7 mg/kg) did not change glomerular filtration rate, fractional reabsorption of sodium, potassium and chloride but results in mild degeneration and vacuolation of the ascending thick portion of the nephron. Higher doses (14.6 mg/kg) result in moderate glomerular sclerosis and severe acute tubular necrosis involving all the nephron segments.Citation47
In mice, arsenic exposure through drinking water (22.5 mg/L) increases urine NAG but not urine albumin (UAlb) excretion. In this model, mice given both arsenic in drinking water and cadmium in food exhibit increases in urine protein and NAG excretion that are markedly higher compared with mice given cadmium or arsenic alone.Citation48
Epidemiological studies in humans
Arsenic has been considered as a therapeutic and poisoning agent since ancient times, and clinical symptoms of acute As toxicity has been described since then, however, arsenic nephrotoxicity was just described 50 years ago when arsenic exposure was associated with development of hemolysis and acute renal failure in industrial workers exposed to this metal; renal biopsies done in these patients showed acute tubular necrosis, renal cortical necrosis, diffuse interstitial fibrosis and further progression to CKD.Citation49,Citation50
More recently, the assessment of health risks associated with exposure to low to moderately elevated levels of arsenic in drinking water has become the subject of considerable interest as some studies have found a direct association between arsenic concentrations in drinking water and adverse health outcomes.
In an ecological-mortality study based on historical data from Utah, Lewis et al.Citation51 found an increased in the mortality ratio associated with renal disease in men but not in women with higher As exposure; Meliker et al.Citation52 found that arsenic in drinking water at levels in excess of 200–300 μg/L was associated with higher mortality rates for diabetes mellitus, cerebrovascular disease and kidney disease in both males and females.
However, the number of epidemiological studies linking chronic-arsenic exposure with markers of renal injury is still scarce and evidence of arsenic as a risk factor for progressive CKD is unclear. Hsueh et al.Citation53 in Taiwan, studied 125 people with GFR ≤60 mL/min and 229 people with normal renal function and found a weak association between urinary levels of As and decreased renal function (r2 = 0.04, p ≤ 0.001), in this study higher As excretion was linked with lower plasma lycopene level, showing that kidney damage is mediated by an increase in the oxidative stress.
Chen et al.Citation54 in Bangladesh, using urinary dipsticks test to detect proteinuria in 10,160 men and women found a higher prevalence of proteinuria in those subjects with higher exposure and higher urinary As excretion; however, incident proteinuria during follow-up visits was not associated with either baseline well As or baseline urinary As. Follow-up of this population showed that an increase >69 µg/L in urinary As excretion raised up the risk for proteinuria.
Zheng et al.Citation23 evaluated the association between inorganic arsenic and albuminuria in American-Indian adults living in rural areas of the United States with low to moderate exposure to As. This population had a high burden for diabetes (49.7%), obesity and albuminuria (30%), and after statistical adjustments for diabetes, urinary Cd excretion, hypertensive medication and systolic blood pressure they found a higher prevalence ratio of albuminuria in subjects with higher As urinary excretion; Robles-Osorio et al.Citation55 in an open-non-diabetic population study done in central Mexico found an increase in the α1-microglobulin urinary excretion as a marker of tubular injury associated with higher urinary arsenic excretion, but this association vanished when data were adjusted for age, body mass index, systolic blood pressure, glucose and uric acid levels.
There is not enough information about the effects of arsenic exposure and renal injury in children, and in the only reported study done by de Burbure et al.Citation56 there was no increase in markers of renal injury in 800 children across Europe with low As exposure.
Some animal data have shown that combined exposure to inorganic arsenic (As) and cadmium (Cd) gives rise to more pronounced renal toxicity than exposure to each of the agents alone,Citation48 and this finding has been corroborated in some epidemiological studies done in humans.Citation57
In China, Hong et al.Citation58 studied 245 subjects co-exposed to arsenic and cadmium, 122 in an arsenic-cadmium polluted area and 123 in a non-polluted area; this group found that the levels of Uβ2MG, UAlb and UNAG in the subjects living in a polluted area were significantly higher than those in the non-polluted area.
Nordberg et al.Citation59 also studied renal dysfunction in 619 persons residing in two metal contaminated areas in China, measurements of Uβ2MG, UNAG, URBP (urinary retinol-binding protein) and UAlb were used as markers of renal dysfunction. A synergic effect between As and Cd was observed as those persons exposed to higher levels of both elements had an increased prevalence of albuminuria and LMW proteinuria than those with higher exposure only to As or Cd alone.
Huang et al.Citation60 evaluated the effect of co-exposure to environmental low-level Cd and As on urinary biomarkers and oxidative stress. Urinary excretion of NAG and the oxidative stress indices (urinary malondialdehyde and 8-hydroxy-2-deoxyguanosine) were positively correlated with Cd and As in urine and these effects were more pronounced with co-exposure to both Cd and As.
Clinical manifestations of arsenic-induced renal disease
The development of acute tubular necrosis with acute renal failure has been reported in patients with systemic toxicity occurring in severe acute arsenic poisoning, and some of these patients develop cortical necrosis and progression to CKD. The precipitating cause of renal injury may be hypotensive shock, direct effects of arsenic on tubule cells and hemoglobinuric or myoglobinuric tubular injury; glomerular damage can result in proteinuria. Acute tubule-interstitial nephritis has also been described as a clinical manifestation of acute As poisoning ().Citation61
Figure 2. Renal biopsy done in a woman with arsenic poisoning and tubulointerstitial nephritis. Biopsy specimen showing a normal glomerulus, extensive interstitial fibrosis with tubular atrophy, and a cellular infiltrate consisting mainly of lymphocytes (Adapted from reference Prasad and Rossi).Citation61
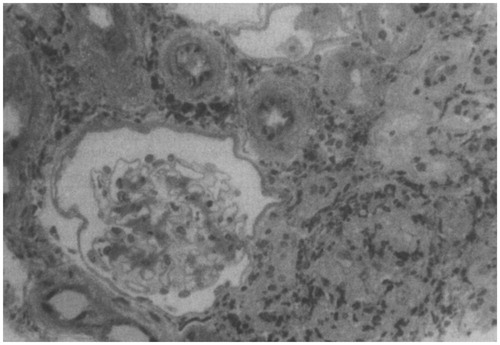
Low molecular weight proteinuria, aminoaciduria, glycosuria and phosphaturia (Fanconi syndrome), as well as progressive deterioration of renal function are clinical characteristics associated with chronic exposure.
In humans, there are no established treatments to decrease As levels in blood or prevent toxicity; however, many therapies and functional foods have been tried in animals in order to prevent arsenic nephrotoxicity. In Chinese Dragon-Li cats exposed to arsenic trioxide to induce renal injury, resveratrol was used to evaluate its effects on arsenic-nephrotoxicity. Resveratrol significantly attenuates the accumulation of arsenic in renal tissues, decreases markers of oxidative stress and shows less morphologic injury and tubular necrosis.Citation62 Other compounds such as naringenin,Citation63 a naturally occurring citrus flavanone; silibinin,Citation64 a naturally occurring plant bioflavonoid found in the milk thistle of Silybum marianum, Pleurotus floridalectin,Citation65 green tea extract,Citation66 Curcuma aromatica leaf extract,Citation67 taurineCitation68 and flaxseed oil,Citation69 have shown promissory effects protecting the kidney against arsenic nephrotoxicity ().
Table 2. Functional foods and prevention of arsenic nephrotoxicity.
In the general population, there is limited awareness of the threats to health associated with As exposure, and several studies have suggested that As mitigation eventually reduces As-associated morbidity, however, this effect may be observed many years after As-free water is provided.Citation70
In conclusion, many pathophysiological mechanisms explain the renal effects related to arsenic exposure, however, the evidence linking arsenic exposure and chronic kidney disease is still scarce and limited to some populations; more studies are needed to know the effects of low to moderate arsenic chronic effects on renal disease.
Declaration of interest
The authors report no conflicts of interest.
References
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28
- Ferraro PM, Costanzi S, Naticchia A, et al. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Public Health. 2010;10:304
- Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084
- Hope JH, Hope BE. A review of the diagnosis and treatment of Ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J Environ Public Health. 2012;2012:835059
- Orth SR, Hallan SI. Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients – Absence of evidence or evidence of absence? Clin J Am Soc Nephrol. 2008;3:226–236
- Siddarth M, Datta SK, Mustafa M, et al. Increased level of organochlorine pesticides in chronic kidney disease patients of unknown etiology: Role of GSTM1/GSTT1 polymorphism. Chemosphere. 2014;96:174–179
- Jelakovic B, Nikolic J, Radovanovic Z, et al. Consensus statement on screening, diagnosis, classification and treatment of endemic (Balkan) nephropathy. Nephrol Dial Transplant. 2014;29:2020–2027
- Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520
- Weaver VM, Kim NS, Jaar BG, et al. Associations of low-level urine cadmium with kidney function in lead workers. Occup Environ Med. 2011;68:250–256
- Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2013;87:969–979
- Ferreccio C, Smith AH, Duran V, et al. Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol. 2013;178:813–818
- Surdu S, Fitzgerald EF, Bloom MS, et al. Occupational exposure to arsenic and risk of nonmelanoma skin cancer in a multinational European study. Int J Cancer. 2013;133:2182–2191
- Chen Y, Graziano JH, Parvez F, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ. 2011;342:2431
- Abhyankar LN, Jones MR, Guallar E, et al. Arsenic exposure and hypertension: A systematic review. Environ Health Perspect. 2012;120:494–500
- Martin-Pardillos A, Sosa C, Sorribas V. Arsenic increases Pi-mediated vascular calcification and induces premature senescence in vascular smooth muscle cells. Toxicol Sci. 2013;131:641–653
- Gribble MO, Howard BV, Umans JG, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol. 2012;176:865–874
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463–466
- Shlipak MG, Day EC. Biomarkers for incident CKD: A new framework for interpreting the literature. Nat Rev Nephrol. 2013;9:478–483
- KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100
- Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581–590
- Salmon AH, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350
- Zheng LY, Umans JG, Tellez-Plaza M, et al. Urine arsenic and prevalent albuminuria: Evidence from a population-based study. Am J Kidney Dis. 2013;61:385–394
- Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep. 2010;12:364–368
- Andersson L, Haraldsson B, Johansson C, et al. Methodological issues on the use of urinary alpha-1-microglobuline in epidemiological studies. Nephrol Dial Transplant. 2008;23:1252–1256
- Dieterle F, Perentes E, Cordier A, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–469
- Pallet N, Chauvet S, Chasse JF, et al. Urinary retinol binding protein is a marker of the extent of interstitial kidney fibrosis. PLoS One. 2014;9:e84708
- Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol (Phila). 2011;49:720–728
- Calatayud M, Barrios JA, Velez D, et al. In vitro study of transporters involved in intestinal absorption of inorganic arsenic. Chem Res Toxicol. 2012;25:446–453
- Argos M, Rathouz PJ, Pierce BL, et al. Dietary B vitamin intakes and urinary total arsenic concentration in the Health Effects of Arsenic Longitudinal Study (HEALS) cohort, Bangladesh. Eur J Nutr. 2010;49:473–481
- Majumdar S, Maiti A, Karmakar S, et al. Antiapoptotic efficacy of folic acid and vitamin B(1)(2) against arsenic-induced toxicity. Environ Toxicol. 2012;27:351–363
- Mitra SR, Mazumder DN, Basu A, et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112:1104–1109
- Liu Z, Boles E, Rosen BP. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J Biol Chem. 2004;279:17312–17318
- Ravera S, Virkki LV, Murer H, et al. Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol. 2007;293:C606–C620
- Carbrey JM, Song L, Zhou Y, et al. Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc Natl Acad Sci USA. 2009;106:15956–15960
- Thomas DJ. Unraveling arsenic – glutathione connections. Toxicol Sci. 2009;107(2):309–311
- He X, Ma Q. Induction of metallothionein I by arsenic via metal-activated transcription factor 1: Critical role of C-terminal cysteine residues in arsenic sensing. J Biol Chem. 2009;284(19):12609–12621
- Liu Y, Liu J, Habeebu SM, et al. Metallothionein-I/II null mice are sensitive to chronic oral cadmium-induced nephrotoxicity. Toxicol Sci. 2000;57:167–176
- Lee TC, Ho IC, Lu WJ, et al. Enhanced expression of multidrug resistance-associated protein 2 and reduced expression of aquaglyceroporin 3 in an arsenic-resistant human cell line. J Biol Chem. 2006;281:18401–18407
- Jimi S, Uchiyama M, Takaki A, et al. Mechanisms of cell death induced by cadmium and arsenic. Ann NY Acad Sci. 2004;1011:325–331
- Tokumoto M, Lee JY, Fujiwara Y, et al. Inorganic arsenic induces apoptosis through downregulation of Ube2d genes and p53 accumulation in rat proximal tubular cells. J Toxicol Sci. 2013;38:815–820
- Lu Y, Yuan H, Deng S, et al. Arsanilic acid causes apoptosis and oxidative stress in rat kidney epithelial cells (NRK-52e cells) by the activation of the caspase-9 and -3 signaling pathway. Drug Chem Toxicol. 2014;37:55–62
- Brazy PC, Balaban RS, Gullans SR, et al. Inhibition of renal metabolism. Relative effects of arsenate on sodium, phosphate, and glucose transport by the rabbit proximal tubule. J Clin Invest. 1980;66:1211–1221
- Li Z, Piao F, Liu S, et al. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp Toxicol Pathol. 2010;62:543–547
- Hossain E, Ota A, Takahashi M, et al. Arsenic upregulates the expression of angiotensin II Type I receptor in mouse aortic endothelial cells. Toxicol Lett. 2013;220:70–75
- Tsukamoto H, Parker HR, Gribble DH, et al. Nephrotoxicity of sodium arsenate in dogs. Am J Vet Res. 1983;44:2324–2330
- Liu J, Liu Y, Habeebu SM, et al. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147:157–166
- Muehrcke RC, Pirani CL. Arsine-induced anuria. A correlative clinicopathological study with electron microscopic observations. Ann Intern Med. 1968;68:853–866
- Gerhardt RE, Hudson JB, Rao RN, et al. Chronic renal insufficiency from cortical necrosis induced by arsenic poisoning. Arch Intern Med. 1978;138:1267–1269
- Lewis DR, Southwick JW, Ouellet-Hellstrom R, et al. Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect. 1999;107:359–365
- Meliker JR, Wahl RL, Cameron LL, et al. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. Environ Health. 2007;6:4
- Hsueh YM, Chung CJ, Shiue HS, et al. Urinary arsenic species and CKD in a Taiwanese population: A case-control study. Am J Kidney Dis. 2009;54:859–870
- Chen Y, Parvez F, Liu M, et al. Association between arsenic exposure from drinking water and proteinuria: Results from the Health Effects of Arsenic Longitudinal Study. Int J Epidemiol. 2011;40:828–835
- Robles-Osorio ML, Perez-Maldonado IN, Martin del Campo D, et al. Urinary arsenic levels and risk of renal injury in a cross-sectional study in open population. Rev Invest Clin. 2012;64:609–614
- de Burbure C, Buchet JP, Leroyer A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: Evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–590
- Buchet JP, Heilier JF, Bernard A, et al. Urinary protein excretion in humans exposed to arsenic and cadmium. Int Arch Occup Environ Health. 2003;76:111–120
- Hong F, Jin T, Zhang A. Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals. 2004;17:573–580
- Nordberg GF, Jin T, Hong F, et al. Biomarkers of cadmium and arsenic interactions. Toxicol Appl Pharmacol. 2005;206:191–197
- Huang M, Choi SJ, Kim DW, et al. Risk assessment of low-level cadmium and arsenic on the kidney. J Toxicol Environ Health A. 2009;72:1493–1498
- Prasad GV, Rossi NF. Arsenic intoxication associated with tubulointerstitial nephritis. Am J Kidney Dis. 1995;26:373–376
- Yu M, Xue J, Li Y, et al. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol. 2013;87:1025–1035
- Mershiba SD, Dassprakash MV, Saraswathy SD. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. 2013;40:3681–3691
- Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep. 2012;39:11201–11216
- Bera AK, Rana T, Das S, et al. Mitigation of arsenic-mediated renal oxidative stress in rat by Pleurotus florida lectin. Hum Exp Toxicol. 2011;30:940–951
- Messarah M, Saoudi M, Boumendjel A, et al. Green tea extract alleviates arsenic-induced biochemical toxicity and lipid peroxidation in rats. Toxicol Ind Health. 2013;29:349–359
- Saxena PN, Anand S, Saxena N, et al. Effect of arsenic trioxide on renal functions and its modulation by Curcuma aromatica leaf extract in albino rat. J Environ Biol. 2009;30:527–531
- Roy A, Manna P, Sil PC. Prophylactic role of taurine on arsenic mediated oxidative renal dysfunction via MAPKs/NF-kappaB and mitochondria dependent pathways. Free Radic Res. 2009;43:995–1007
- Rizwan S, Naqshbandi A, Farooqui Z, et al. Protective effect of dietary flaxseed oil on arsenic-induced nephrotoxicity and oxidative damage in rat kidney. Food Chem Toxicol. 2014;68C:99–107
- Pi J, Yamauchi H, Sun G, et al. Vascular dysfunction in patients with chronic arsenosis can be reversed by reduction of arsenic exposure. Environ Health Perspect. 2005;113:339–341
