Abstract
Background: Patients undergoing hemodialysis (HD) present altered levels of appetite hormones such as acyl-ghrelin (orexigenic) and obestatin (anorexigenic), which may contribute to anorexia. Physical exercise may affect these hormones and improve appetite in these patients. Objectives: The objective of this study is to evaluate the effects of a resistance exercise program in appetite hormones, body composition, and nutritional status in HD patients. Design: Intervention study with the control group. Subjects: Fifty-two patients on regular HD program were enrolled into two groups: 37 patients performed exercises (56.7% male, 45 ± 12.8 years, 57 (9–192) months on HD) and 15 patients comprised the control group (66.7% men, 50 ± 10.6 years, 57 (11–153) months on HD). Measurements: Exercise program (performed with elastic bands and ankle cuffs in both lower limbs) was supervised three times a week during 6 months (72 sessions). Patients had their blood drawn in a regular HD day after overnight fasting, before and after 6 months of exercise program. Obestatin, acyl-ghrelin, routine biochemical parameters, quality of life, and anthropometric data were collected and analyzed before and after 6 months. Results: After 6 months of exercise, obestatin levels reduced [from 3.0 ng/mL (2.3–3.4) to 1.9 ng/mL (0.6–3.4)] and acyl-ghrelin levels increased [from 21.5 pg/mL (1.3–77.7) to 37.2 pg/mL (16.7–94.1)] and the control group presented no significant differences in both plasma levels of hormones. Body composition and physical functional assessed by SF-36 and albumin levels (3.7 ± 0.3 to 3.9 ± 0.2, p < 0.05) improved after exercises. Conclusion: Six months of resistance exercises contributed to changes in plasma appetite hormones, body composition, and nutritional status in hemodialysis patients.
Introduction
Ghrelin is a 28 amino acid hunger-stimulating peptide produced mainly by X/A-like cells of oxyntic mucosa and it was identified as the natural ligand of the growth hormone secretagogue receptor (GHSR) in 1999 by Kojima et al.Citation1 Ghrelin must be acylated (by ghrelin O-acyltransferase) at the serine 3 level in order to become active (acyl-ghrelin) and this form is quite unstable and rapidly metabolized into the des-acylated form (des-acyl ghrelin). The active form is essential for stimulation of growth hormone (GH) secretion from the pituitary and stimulation of food intake.Citation2,Citation3
In addition to the ghrelin mature peptide, Zhang et al.Citation4 identified a 23-amino acid peptide from the same gene of acyl-ghrelin, obestatin, which acts in the central nervous system through the arcuate and paraventricular nuclei of the hypothalamus activating neuropeptide that suppress food intake.
Studies about acyl-ghrelin and obestatin in chronic kidney disease (CKD) patients are of interest because these compounds may be implicated with reduced appetite, loss of weight, and malnutrition.Citation5 Although controversial results, studies have shown alteration in acyl-ghrelin and obestatin levels in CKD patients.Citation5,Citation6
There are several interventions to treat the loss of appetite and malnutrition in HD patients, and physical exercises have been considered as a complementary strategy. Studies in non-CKD patients have presented that the levels of acyl-ghrelin would increase with physical exercises.Citation7–10 Studies about the effects of physical exercises on obestatin levels are extremely rare in the literature. One study showed that plasma obestatin levels decreased after intervention with physical exercise; on the other hand, another study found no changes after intervention with physical exercises.Citation11,Citation12 To date, there is no publication about the effects of resistance exercise in acyl-ghrelin and obestatin levels in CKD patients undergoing HD. Therefore, the purpose of this study was to analyze the effect of resistance exercise training program (RETP) on acyl-ghrelin and obestatin levels in patients undergoing hemodialysis (HD). Additionally, quality of life, functional capacity, and nutritional status were analyzed.
Design and methods
Subjects
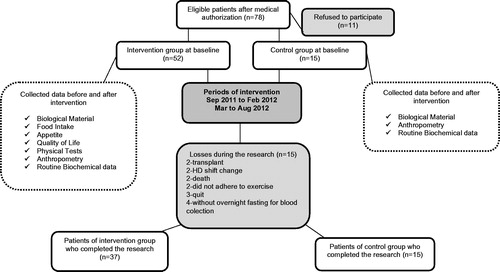
This longitudinal, retrospective study enrolled 67 CKD patients undergoing HD. The sample size was calculated to know the number of patients required in the exercise and the control group to have sufficient statistical power. The sample of 35 patients in the exercise group and 10 patients in the control group was determined for the significance p = 0.05 and a test power of 0.8.
Thirty-seven patients (56.7% men) were designated to the exercise group and 15 patients (66.7% men) were allocated to the control group. The stages and the number of patients throughout the study are shown in the flowchart (). The inclusion criteria were age >18 years and patients were on maintenance dialysis for at least 6 months. Patients with inflammatory and autoimmune diseases, cancer, acquired immunodeficiency syndrome (AIDS), amputated limbs, bariatric surgery, under regular exercise, taking catabolizing drugs, and patients with catheter access for hemodialysis were excluded. Patients who complied with less than 75% (less than 54 exercise sessions) of the RETP were excluded. The dialysis duration was 3–4.5 h per session, three times per week, with a blood flow greater than 250 mL/min and a dialysate flow of 500 mL/min. The etiologies included hypertension (71.2%), chronic glomerulonephritis (11.5%) unknown causes (9.6%), diabetes (5.8%), and obstructive uropathy (1.9%). This study was conducted according to the guidelines established in the Declaration of Helsinki and Ethics Committee approved procedures involving human subjects/patients (number 073/10). Written informed consent was obtained from all subjects/patients. Ethics Committee of the Medical School of the Federal Fluminense University approved the study.
Exercise training program
The exercises were performed during the first 2 h of hemodialysis, three times per week for 6 months (72 sessions). The exercises were performed in both lower limbs, and the intensity was based on the 1-Repetition Maximum Test (1RM).Citation13 The initial intensity was 60% of 1RM (performed according to the equivalent kilograms specified in the manufacturer's manual) because CKD patients are mostly debilitated. According to the performance of the patients, the intensity of the exercise reached 70% of 1RM after 12 sessions. The patients performed the exercises with ankle-cuffs and elastic bands (Theraband®, Hygenic Corporation, Akron, OH), and the intensity varied depending on the color of the bands. The exercises were administered by a physical educator following the Cheema’sCitation14 adapted protocol: (1) the first exercise performed was a knee extension from 90° to 0°, with the patient remaining in the 0° position for 5 s (an isometric contraction) and returning to the starting position after 5 s. Each contraction phase of exercise (concentric and eccentric) was performed in approximately 3 s. (2) The second exercise consisted of a triple flexion, followed by extension of the lower limbs. The patient held a flexion thigh, knee, and ankle, with the elastic band placed at the level of the metacarpals, and the patient held a triple extension of thigh, knee and ankle. (3) In a co-isometric contraction, the patient performed a leg extension against resistance located in the distal third of the thigh for 10 s. (4) The patient performed a hip joint flexion of the lower limb by rising to their functional limit (the leg that did not perform the movement was kept resting on a bench with knee and thigh flexed).
Resistance was located in the distal third of the leg at the level of the malleoli. The patient rested 1 min between four sets of 10 repetitions, and 3 min between the exercise categories. The 1RM test was performed every 12 sessions, and the load was added according to their results. All exercises were performed during HD sessions, and the patients remained seated while performing the exercises. The load used in the exercise with elastic band ranged from 1.6 to 10.0 kg, and the load of exercises performed with ankle-cuffs ranged from 1.0 kg to 12.0 kg. Exercise compliance was assessed every week using an adherence list kept by the trainers, and adherence was defined as the number of training sessions attempted divided by the number offered × 100%.
Analytic procedures and biochemical analysis
Blood samples were drawn at the baseline and after 72 sessions of RETP from each subject in the morning, after overnight fasting and prior to the dialysis session. The blood was collected in Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) (1.00 mg/dL) as an anticoagulant. The blood was centrifuged (15 min, 3000 × g, 4 °C) to obtain the plasma and stored in polypropylene tubes at −80 °C until analysis. The levels of hormones were both analyzed using enzyme immunoassay kits. Obestatin plasma levels were analyzed with a specific commercial kit using a specific protocol designed by the fabricant (Host: Rabbit Bachen®, San Carlos, CA, catalogue number S-1285, protocol II, Std.Ab1hr.BtON).
Acyl-ghrelin levels were analyzed with a commercial kit that specifically detect acylated fraction (Human acylated Ghrelin EIA – SPI Bio, Montigny, France, catalogue number A05106). Plasma was diluted with buffer (1:5) according to instructions of the manufacturer.
Routine biochemical parameters, including albumin, creatinine, and hemoglobin, were measured for all patients according to standard methods at a routine clinical laboratory. The kinetic index of dialysis adequacy (Kt/V) was calculated from the values of blood urea nitrogen, pre- and post-dialysis, body weight, and dialysis duration using a logarithmic formula.Citation15
Anthropometric parameters
The following anthropometric parameters were measured: body weight (kg), height (m), waist circumference (WC, cm), and skinfold measurement (mm) at four standard sites (biceps, triceps, subscapular, and suprailiac) using a Lange Skinfold Caliper (Cambridge Scientific Products, Cambridge, MA). Arm muscle area (AMA) was calculated according to the following formula: (MAC (cm) – п × triceps skin fold thickness (cm))2/4 × п – n, where n = 10 for males and 6.5 for females. Body fat was calculated from the skinfold measurement and the percentage of body fat was calculated. The fat-free mass (kg) was calculated by subtracting the fat mass (kg) from the body weight (kg). The body mass index (BMI) was calculated as the body weight (kg) divided by the squared stature (meter) and was used to assess the nutritional status according to the WHO.Citation16 A trained staff member performed all the measurements after the hemodialysis session.
Nutritional status
The nutritional status was evaluated by trained professionals using the adapted Subjective Global Assessment (SGA) proposed by Kalantar et al.,Citation17 which is a fully quantitative scoring system (the dialysis malnutrition score) consisting of seven variables: weight change, dietary intake, gastrointestinal symptoms, functional capacity, comorbidity, subcutaneous fat, and signs of muscle wasting. Each component was assigned a score from 1 (normal) to 5 (very severe). The sum of all seven components in this malnutrition score lies between 7 (normal) and 35 (severely malnourished). Patients were classified as well nourished, at nutritional risk, moderate malnourished, severe malnourished, and very severely malnourished.
SF36 – quality of life scoring
The SF-36 is a multi-purpose, short-form health survey with 36 questions. The SF-36 is used to assess the quality of life in many diseases including CKD and it is validated in HD patients.Citation18 The questionnaire has two main dimensions (mental health and physical health) and eight scales or components, i.e., physical function, role physical, body pain, vitality, general health, mental health (not to be confused with the dimension under the same designation), role mental, and social function. The scales vitality and general health are part of both the mental and physical health dimensions. Each of the eight scales is rated 1–5, which contribute to the scoring of these scales. The SF-36 scores of each of the two dimensions are based on the mathematical averaging of the scores of five scales, and the questionnaire is validated and translated to Portuguese.Citation18,Citation19
Statistical analysis
The distribution of the variables was analyzed using the Kolmogorov–Smirnov or Shapiro–Wilk tests as appropriate. The normally distributed variables were expressed as mean ± standard deviation and not normally distributed variables were expressed as median (quartiles). The differences of the variables were analyzed using non-parametric tests (paired tests, Wilcoxon W, Mann–Whitney U, or McNemar) or parametric tests (independent samples T-test or paired samples T-test). The correlation between variables was analyzed using Spearman or Pearson coefficients depending on the distribution of the variable.
Plasma obestatin and acyl-ghrelin levels’ change was based on the following formula: [(levels before RETP − levels after RETP)/levels before RETP] which was used to perform statistical analysis. Since the change in plasma obestatin and acyl-ghrelin levels presented normal distribution (Shapiro–Wilk test, p 0.001), the linear regression model was used to analyze the relationship of the dependent variables (change in obestatin and acyl-ghrelin) and the explanatory variables, which were also analyzed according to their change. The analyses were performed in R version 2.15.1 (SPSS Inc., Chicago, IL) and a significance level of 5% was accepted.
Results
Fifty-two patients undergoing hemodialysis completed the study. The group that performed exercises consisted of 37 patients of which 21 were men, aged 45.5 ± 10.8 years and 16 were women aged 44.3 ± 15.4 years and time on dialysis for both men and women was 57 (9–192) months on HD. The control group consisted of 15 patients including 10 men aged 50 ± 12.7 years and 5 women aged 49.4 ± 4.8 years that have been performing HD for 57 (11–153) months. There was no significant difference of age and time on HD between the groups.
Biochemical and anthropometric parameters are presented in . Baseline acyl-ghrelin level of the control group was significantly higher than the exercise group, but there were no changes in hormones levels (acyl-ghrelin and obestatin) after 6 months in the control group. After intervention with exercises the obestatin levels decreased and acyl-ghrelin increased significantly. About 30% of the patients who underwent exercise presented creatinine serum levels lower than 10 mg/dL, and, according to hemoglobin values, 46% of patients presented anemia. In the control group, 81.8% of patients had creatinine below the values, recommended for patients on hemodialysis (10 mg/dL) and anemia was present in 27% of patients (). Before exercise, 65.2% of patients presented albumin level <3.8 g/dL that was reduced to 37.5% after intervention with exercise (p < 0.05).
Table 1. Comparison of biochemistry and anthropometric data before and after 6 months of resistance training exercise program (RETP) between exercise and control group.
The anthropometric parameters showed that the control group presented gain of fat mass and the exercise group increased fat-free mass after intervention (). In the exercise group, 16.6% of the patients presented BMI <18.5 kg/m2, approximately 45% presented adequate weight (18.4 kg/m2 <BMI <24.9 kg/m2), 32.4% were overweight (25 kg/m2 <BMI <30 kg/m2), and 5.4% were obese (30 kg/m2 <BMI <34.9 kg/m2). In the control group, 40% of the patients presented adequate BMI (18.4 kg/m2 <BMI <24.9 kg/m2), 53.4% of the patients were overweighed (25 kg/m2 <BMI <30 kg/m2), and 6.6 % were obese. According to WHO classification, the percentage of patients with underweight, normal weight, overweight, and obesity did not change significantly in both groups.
The malnutrition score, Subjective Global Analysis (SGA), was only performed in the exercise group and, before RETP, 5.8% of the patients were well nourished, 67.3% were at nutritional risk, and 26.9% were moderate malnourished, and after RETP 27.3% of the patients were well nourished, 63.6% presented nutritional risk, and 9.1% were moderated malnourished (p < 0.0001).
Quality of life data displayed in shows that among the eight components of SF-36 assessed before and after 6 months, only physical role improved significantly in the intervention group. Although the other components have not improved significantly, there was a trend towards improvement of these components. The SF-36 components’ of the control group did not change during the study period and the physical aspects after 6 months of exercise in the intervention group were significantly better when compared with the control group values ().
Table 2. Quality of life evaluation before and after 6 months of exercise and control group.
The linear regression model revealed that the plasma acyl-ghrelin change was associated with arm muscle area and that the reduction of plasma obestatin levels was associated with decreased body fat and increased albumin and AMA ().
Table 3. Simple linear regression model examining the effects of the variables that presented significant changes after resistance exercise training program of exercise group on acyl-ghrelin and obestatin changes independent of resistance exercise training program.
Discussion
The results of the present study showed that acyl-ghrelin and obestatin plasma levels increased significantly in HD patients that performed 6 months of RETP unlike the patients in the control group. In addition, patients that performed 6 months of resistance exercise improved parameters such as serum albumin, body composition, nutritional status, and quality of life. In the same period, the control group did not present changes in hormones plasma levels, quality of life, and gained fat mass.
To the best of our knowledge, there is no study about the effects of resistance exercise on plasma acyl-ghrelin and obestatin levels in HD patients. Studies in non-CKD patients have shown that exercise (acute or long-term) can alter the levels of gut peptides, but data are controversial. Some studies have revealed increase in total ghrelin levels during acute aerobic exercise.Citation20,Citation21 On the other hand, studies have shown no change in ghrelin levels after aerobic exercise,Citation22,Citation23 however, these studies measured total ghrelin but not acyl-ghrelin or obestatin. Guelfi et al.Citation8 observed no changes in acyl-ghrelin levels of adults (male) after 12 weeks of aerobic or resistance training; however, volunteers who performed running (aerobic exercise) presented a trend for increase in acyl-ghrelin levels.Citation10 Additionally, a studyCitation9 that compared healthy males and females showed compensatory increases in acyl-ghrelin levels in women but not men after 4 d of exercise session conducted on a treadmill.
A small number of studies have investigated the effects of exercise in plasma obestatin, and some of them have found no effect of exercise in non-CKD patientsCitation12; although one study found decreased plasma obestatin levels after intervention with exercise in rats.Citation11 Obestatin is associated with diverse biological functions of gastrointestinal and adipose tissues,Citation24 and here we presented data showing that the change of plasma obestatin levels after RETP was related to body composition (AMA and body fat) and nutritional status (albumin).
In addition to peptides changes, the studied HD patients also presented serum albumin, BMI, fat-free mass, and ASG score improved after RETP. In fact, it is known that albumin levels are a good predictor of nutritional status of HD patients, and it has been shown that each 1 g/dL fall in serum albumin level is associated with a 39% increase in risk of cardiovascular death.Citation25 Accordingly, previous results of this study showed that PEW reduced significantly in the intervention group mainly due to albumin levels, BMI and AMA.Citation26 Accordingly, Johansen et al.Citation27 reported that resistance training improved quadriceps muscle area in HD patients similar to other studies with HD patients that confirmed important results about resistance training and improved knee strength, lean body mass, and physical capacity.Citation28–30 In the present study, patients who underwent RETP also improved the role physical of SF-36 after intervention while the control group did not present any changes in quality of life. In fact, studies have reported positive outcomes of intervention studies with exercise in quality of life, e.g., HD patients that performed resistance exercise improved significantly their mental health,Citation30 and resistance combined with aerobic exercise showed improvement of mental and physical components.Citation31 The improvement of quality of life after intervention with resistance exercise is well established and taken together these results highlight the positive influence of exercise in quality of life of HD patients.Citation27,Citation28
Nutritional strategies to increase appetite such as caloric supplementation and appetite stimulants have been largely unsuccessful in CKD patientsCitation5; therefore, alternative therapies like physical exercise can be a new strategy to increase appetite in CKD patients. Indeed, it has been reported that the low acyl-ghrelin levels in HD patients could be associated with reduced food intake and poor nutrition.Citation6 Therefore, exercise seems to be a potential intervention to regulate appetite hormones.Citation32
One of the limitations of this study is that we did not analyze energy intake due to great bias of food records since this tool is not completely reliable to evaluate caloric intake.Citation33 Thus, it seems more accurate to measure and analyze the levels of hormones related to appetite and markers of nutritional status as in hemodialysis patients the accurate assessment of energy intake is crucial in evaluating clinical status, nutritional interventions, and health outcomes. One of the major problems in dietary assessment is the inaccuracy in reporting dietary intake, i.e., one study showed that 65% of the HD patients underreported their energy intake.Citation33 Another limitation of this study is that we did not perform SGA of the control group due to logistical issues.
Here we presented data showing that plasma acyl-ghrelin increased and obestatin decreased after 6 months of intervention with RETP, and body composition and nutritional status were related to these changes. We also observed increase in fat-free mass improvement of the malnutrition score and serum albumin. These results bring perspectives for future studies about exercise and appetite hormones in hemodialysis patients.
Practical application
Alteration in appetite hormone levels is implicated with reduced appetite and malnutrition in patients with CKD, and exercises seem to be a good intervention to balance acyl-ghrelin and hormone levels and improve quality of life of these patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660
- Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325
- Van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–457
- Zhang JV, Ren P-G, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310(5750):996–999
- Mafra D, Jolivot A, Chauveau P, et al. Are ghrelin and leptin involved in food intake and body mass index in maintenance hemodialysis? J Ren Nutr. 2010;20(3):151–157
- Mafra D, Guebre-Egziabher F, Cleaud C, et al. Obestatin and ghrelin interplay in hemodialysis patients. Nutr Burbank Los Angel Cty Calif. 2010;26(11–12):1100–1104
- Deighton K, Barry R, Connon CE, Stensel DJ. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur J Appl Physiol. 2013;113(5):1147–1156
- Guelfi KJ, Donges CE, Duffield R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism. 2013;62(2):235–243
- Hagobian TA, Sharoff CG, Stephens BR, et al. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R233–R242
- Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM. Influence of running and walking on hormonal regulators of appetite in women. J Obes. 2012;2012:730409
- Ghanbari-Niaki A, Jafari A, Abednazari H, Nikbakht H. Treadmill exercise reduces obestatin concentrations in rat fundus and small intestine. Biochem Biophys Res Commun. 2008;372(4):741–745
- Manshouri M, Ghanbari-Niaki A, Kraemer RR, Shemshaki A. Time course alterations of plasma obestatin and growth hormone levels in response to short-term anaerobic exercise training in college women. Appl Physiol Nutr Metab. 2008;33(6):1246–1249
- Brown LE, Weir JP. JEP Online. ASEP Proc Recomm Accurate Assess Muscular Strength Power. 2001;4(3):1–21
- Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): A randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–1601
- Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int. 1985;28(3):526–534
- World Health Organization. Obesity: Preventing and managing the global epidemic: Report of a WHO Consultation. Geneva, Switzerland: World Health Organization. WHO Technical Report Series 894; 2000
- Kalantar-Zadeh K, Kleiner M, Dunne E, Lee GH, Luft FC. A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant. 1999;14(7):1732–1738
- Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang H, Lazarus JM. Quality-of-life evaluation using Short Form 36: Comparison in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2000;35(2):293–300
- Ciconelli RM, Ferraz MB, Santos W. Rev Bras Reumatol. Tradução Para Língua Port E Validação Quest Genérico Aval Qual Vida SF-36 Bras SF-36. 1999;39(3):143–150
- Christ ER, Zehnder M, Boesch C, et al. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. Eur J Endocrinol. 2006;154(3):397–403
- Russel RR, Willis KS, Ravussin E, Larson-Meyer ED. Effects of endurance running and dietary fat on circulating ghrelin and peptide YY. J Sports Sci Med. 2009;8(4):574–583
- Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J Sports Sci. 2007;25(6):635–642
- Dall R, Kanaley J, Hansen TK, et al. Plasma ghrelin levels during exercise in healthy subjects and in growth hormone-deficient patients. Eur J Endocrinol. 2002;147(1):65–70
- Zhang JV, Jahr H, Luo C-W, et al. Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Mol Endocrinol Baltim Md. 2008;22(6):1464–1475
- Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62(6):2238–2245
- Moraes C, Marinho SM, da Nobrega AC, et al. Resistance exercise: A strategy to attenuate inflammation and protein-energy wasting in hemodialysis patients? Int Urol Nephrol. 2014;46(8):1655–1662
- Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006;17(8):2307–2314
- Chen JLT, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult hemodialysis patients: A randomized pilot trial. Nephrol Dial Transplant. 2010;25(6):1936–1943
- Segura-Ortí E, Kouidi E, Lisón JF. Effect of resistance exercise during hemodialysis on physical function and quality of life: Randomized controlled trial. Clin Nephrol. 2009;71(5):527–537
- Segura-Ortí E, Rodilla-Alama V, Lisón JF. Physiotherapy during hemodialysis: Results of a progressive resistance-training programme. Nefrol Publ Soc Esp Nefrol. 2008;28(1):67–72
- Oh-Park M, Fast A, Gopal S, et al. Exercise for the dialyzed: Aerobic and strength training during hemodialysis. Am J Phys Med Rehabil. 2002;81(11):814–821
- Mackelvie KJ, Meneilly GS, Elahi D, Wong ACK, Barr SI, Chanoine J-P. Regulation of appetite in lean and obese adolescents after exercise: Role of acylated and desacyl ghrelin. J Clin Endocrinol Metab. 2007;92(2):648–654
- Mafra D, Moraes C, Leal VO, Farage NE, Stockler-Pinto MB, Fouque D. Underreporting of energy intake in maintenance hemodialysis patients: A cross-sectional study. J Ren Nutr. 2012;22(6):578–583