Abstract
Metabolic acidosis is a common feature in chronic renal failure patients, worsening progressively as renal function declines. There are conflicting data in hemodialysis (HD) patients with regard to acidosis, alkalosis and mortality. In HD patients, cognitive impairment, depression, sleep disorders and impaired quality of life are very common. Besides, these conditions are related with increased morbidity and mortality. However, no previous study investigated the relationship between pH, venous bicarbonate and anion gap with depression, sleep problems and cognitive function in HD patients. In this study we investigated these relationships. In total, 65 HD patients were included. The demographic parameters and laboratory parameters including bicarbonate, pH and anion gap was measured for all patients. Depressive symptoms, sleep quality and cognitive function, were measured by Beck depression inventory, The Pittsburgh Sleep Quality Index and by Mini Mental State Examination, respectively. We found that, sleep quality but not cognitive function or depression was independently related with venous pH and bicarbonate. Anion gap has no independent relationship with sleep quality, cognitive function and depression. In conclusion, metabolic acidosis and bicarbonate levels were independently related with sleep quality in HD patients. However, there was no association between metabolic acidosis and bicarbonate levels with cognitive function and depression.
Introduction
Metabolic acidosis is a common feature in chronic renal failure patients and worsens progressively as renal function declines. Hyperchloremia is partly responsible for acidosis in the earlier stages but with the progressive decline in renal function as in patients with end-stage renal disease, pure high anion gap acidosis appears.Citation1,Citation2 In hemodialysis (HD) patients, acidosis has detrimental effects in terms of renal osteodystrophy, inflammation and nutritional aspects. Thus, although it remains controversial, it was recommended to maintain serum bicarbonate level >22 mEq/L.Citation3,Citation4 In contrast, there is evidence that there was inverse association between small decreases in serum bicarbonate and mortality.Citation5 Indeed, it was shown that moderate predialysis acidosis was associated with better nutritional status and lower relative risk for mortality and hospitalization.Citation6 To complicate the things further, very recent evidence suggests that low bicarbonate levels were associated with worsening of kidney function.Citation7,Citation8
Not only observational studies, interventional studies have also shown conflicting results regarding the correction of acidosis and outcomes. In one study, it was shown that mortality was lowest in levels between 17 and 23 and increased at both ends of the spectrum (<17 and >27 mEq/L). However, after adjustments for various parameters for malnutrition inflammation complex syndrome (MICS),
concentration of ≥22 mEq/L was associated with less risk for death, whereas
levels <22 mEq/L were associated with the highest death risk. Indeed, it was suggested that association between metabolic acidosis and alkalosis versus survival that maybe influenced by clinical conditions or other dominating characteristics.Citation4
In HD patients, cognitive impairment, depression, sleep disorders and impaired quality of life are very common and related with increased morbidity and mortality.Citation9,Citation10 As suggested above, metabolic acidosis and alkalosis versus survival may be influenced by clinical conditions or other dominating characteristics related also with morbidity and mortality such as depression, decreased sleep quality and cognitive function. However, no previous study investigated the relationship between pH, venous bicarbonate and anion gap with depression, sleep problems and cognitive function in HD patients. In this study, we investigated these relationships.
Materials and methods
The observational study was performed on regular HD patients with end-stage renal disease receiving HD therapy thrice weekly. The study included 65 HD patients. The study was in accordance with the declaration of Helsinki, and Selcuk University ethics committee approved the study and informed consent was obtained before enrollment.
The inclusion criteria for the patient enrollment was described as: patients >18 years of age with a minimum 1-year HD duration, patients without acute coronary syndrome in last 3 months, patients not taking antidepressants, patients without dementia and Alzheimer disease and patients who wanted to participate in the study. All patients were clinically euvolemic, no patient was under oral alkali therapy and no patients were using sevelamer during the study period. None of the patients was on oral bicarbonate replacement or had any process such as diarrhea or vomiting that could impact the acid–base status at the time of the study.
The exclusion criteria were as follows: patients with an ischemic leg ulcer or peripheral revascularization procedures within last 6 months, patients with restless leg syndrome, patients with Alzheimer disease and patients who were taking antidepressants, patients with known obstructive sleep apnea and patients with chronic obstructive lung disease. Additionally, patients who had suffered from acute coronary syndrome, myocardial infarction, angina pectoris, or coronary revascularization procedure (coronary stent replacement and coronary artery by-pass graft surgery) within last 3 months were excluded. Coronary artery disease was defined as the presence of previous myocardial infarction, angina pectoris or coronary revascularization procedure.
After being given a brief explanation, all patients underwent the following procedures: history taking, physical examination, blood pressure measurement before dialysis session. During the anamnesis procedure, demographic and clinical characteristics, measurements of depressive symptoms, sleep quality and cognitive function were performed. To sustain validity, the laboratory parameters were taken after 2 d off dialysis for all patients. The fasting laboratory parameters including predialysis blood urea nitrogen and creatinine, hemoglobin, albumin, sodium, potassium, calcium, phosphorus, chloride, high sensitive C-reactive protein (hs-CRP), calcium and phosphorus, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride and intact parathyroid hormone (iPTH) were measured before the beginning of session. Postdialysis serum urea nitrogen levels, used to calculate urea reduction ratio and HD dose were also measured. For the calculation of pH and bicarbonate, venous blood gas analysis was performed before HD session. Calculation of anion gap was based on the following formula: [Na++K+]-[Cl−+] where Na+ denotes for sodium, K+ denotes for potassium, Cl− denotes for chloride,
denotes for bicarbonate.
The measurement of cognitive function, depressive symptoms and sleep quality was measured by The Standardized Mini Mental State Examination (SMMSE),Citation11 Beck depression inventory (BDI)Citation12 and by The Pittsburgh Sleep Quality Index (PSQI),Citation13 respectively.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Evanston, IL). Results were considered statistically significant if two-tailed p-value was <0.05. Data are shown as mean ± standard deviation. Data were checked for normality. To compare PSQI, BDI and SMMSE among venous bicarbonate quartiles; Kruskal–Wallis test was used. For the post hoc analysis Bonferroni corrected Mann–Whitney U test was used. To analyze the univariate associations between non-normally distributed variables, Spearman’s correlation analysis was used. Additionally, several multivariate stepwise linear regression analyses were performed to assess independent parameters related with logarithmically converted PSQI score.
Results
Initially, 90 patients were recruited. Two patients with acute coronary syndrome, one patient with limp amputation, one patient with Alzheimer disease, five patients taking antidepressants, four patients with restless leg syndrome, two patients with obstructive sleep apnea, four patients with chronic obstructive lung disease, six patients who did not want to participate, were excluded. In remaining 65 HD patients the study was conducted. The etiologies of end-stage renal disease were as follows:
Diabetes mellitus (n = 9), hypertension (n = 18), glomerulonephritis (n = 7), amyloidosis (n = 2), vesicourethral reflux and pyelonephritis (n = 5), nephrolithiasis (n = 4), polycystic kidney disease (n = 4), preeclampsia (n = 1), and unknown (n = 15). The baseline demographic characteristics of the patients are shown in . The clinical and laboratory data of 65 hemodialysis patients were shown in .
Table 1. Baseline demographic characteristics of 65 hemodialysis patients.
Table 2. The clinical and laboratory data of 65 hemodialysis patients.
Table 3. Serial stepwise multiple linear analyses of factors independently associated logarithmically converted PSQI score.
The mean PSQI, BDI, SMMSE scores were 10.4 ± 3.81, 11.1 ± 8.13 and 25.0 ± 2.92, respectively. The PSQI score was correlated with venous pH (ρ = −0.469, p < 0.0001), venous bicarbonate (ρ = −0.538, p < 0.0001), sodium (ρ = −0.281, p = 0.023), fasting blood glucose (ρ = +0.474, < 0.0001), albumin (ρ = −0.362, p = 0.003), total cholesterol (ρ = 0.368, p = 0.003) and triglyceride: (ρ = 0.338, p = 0.007).
Beck Depression Score was correlated with venous bicarbonate (ρ = −0.289, p = 0.020), age (ρ = −0.401, p = 0.001), SMMSE score (ρ = −0.387, p = 0.001) and with blood urea nitrogen: (−0.273, p = 0.028).
The SMMSE score was correlated with fasting blood glucose (ρ = −0.313, p = 0.011) and with BDI score (ρ = −0.387, p = 0.001).
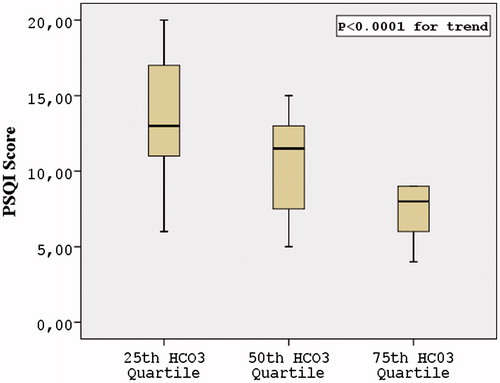
The 25th, 50th and 75th of venous bicarbonate quartiles were 17.7, 19.1 and 19.95 mmol/L, respectively. The comparison of PSQI score, SMMSE score and BDI score among 25th, 50th and 75th of venous bicarbonate quartiles showed that only PSQI score, (p < 0.0001 for trend) (), but not SMMSE score (p = 0.982) and BDI score (p = 0.176) were statistically different. Post hoc analysis of PSQI score showed that PSQI score was different between 25th and 75th quartiles (p < 0.0001) and between 50th and 75th quartile (p = 0.003) but not between 25th and 50th quartile (p = 0.101) ().
Figure 1. Comparison of The Pittsburgh Sleep Quality Index Score among venous bicarbonate quartiles.
To determine the independent factors related with logarithmically converted PSQI score, a serial stepwise linear regression analyses were performed to avoid co-linearity. Serial stepwise multiple linear analyses of factors independently associated logarithmically converted PSQI score were shown in . In the first model, the independent factors including age, gender, marital status, economical status, body mass index, HD duration, smoking status, presence of diabetes and coronary artery disease, albumin, phosphorus, venous pH, BDI score and SSMSE score showed that pH and coronary artery disease were independently related with log PSQI score. In the second model using same independent variables plus venous bicarbonate minus pH showed that venous bicarbonate and coronary artery disease were independently related with log PSQI score. In Model 3, same independent variables as in Model 1 plus anion gap minus pH and venous bicarbonate showed that only coronary artery disease was related to log PSQI score.
Discussion
In this study, we investigated the relationship between venous pH, venous bicarbonate and anion gap with depressive symptoms, sleep quality and cognitive function. We have demonstrated that venous pH and bicarbonate were independently associated with sleep quality as evaluated by PSQI score. There were no associations between cognitive function and depression with pH, bicarbonate and anion gap. To the best of our knowledge, these data are novel and were not demonstrated before.
As a major finding of our study, we showed that as pH and bicarbonate increases, PSQI score decreases or vice versa (a negative correlation) meaning that sleep quality increases as pH and bicarbonate increases. To say in another way, higher venous bicarbonate and pH may have a permissive role for good sleep. But what are the mechanisms underlying sleep quality, pH and bicarbonate? Currently we do not know the answer but speculations can be made.
First, obstructive sleep apnea (OSA) may be one of the explanations. The prevalence of sleep apnea in HD patients is high and it has an adverse effect on the quality of life in these patients.Citation14 Indeed, Tada et al.Citation15 demonstrated that the number of OSA events per hour was significantly and inversely correlated with serum bicarbonate in HD patients and the authors concluded that apart from other factors, metabolic acidosis is a predictor of OSA. Unfortunately, in this study although patients with OSA were excluded, no definitive tests were performed including polysomnography. Indeed, it was reported that nocturnal HD decreased serum creatinine concentration, increased serum bicarbonate concentration and improved not only central sleep apnea but also OSA in patients with chronic renal failure. These results might support that uremic toxins and metabolic acidosis had important roles in the occurrence of OSA.Citation16
Second, metabolic acidosis may directly result in upper airway closure and leading to apnea which results in decreased sleep quality. Sharp et al.Citation17 showed that metabolic acidosis induced by acetazolamide converted central apnea to obstructive apnea, and they suggested that metabolic acidosis might stimulate the activity of the bellows muscles (diaphragm, intercostals, scalene) to a greater extent than it stimulates the upper airway muscles, resulting in upper airway closure. In addition to these facts HD patients are already predisposed to upper airway occlusion due to airway edema, which is associated with fluid overload, and to reduced muscle tone, which is associated with uremic toxins.Citation18
The other explanation between metabolic acidosis, bicarbonate and sleep quality may be explained in the context of heart rate variability (HRV). Diminished HRV is common in HD patients.Citation19 In patients with end-stage renal disease, a higher bicarbonate level is associated with a modest linear improvement in HRV variables suggesting that attention to acidosis could partially normalize the patterns of HRV variability in these populations.Citation20 Unfortunately, we did not perform HRV analysis.
The role of coronary artery disease with disturbed sleep is worth to speak. Jung et al.Citation21 demonstrated that sleep apnea syndrome is associated with coronary artery calcification in HD patients. Unruh et al.Citation22 showed that one of the independent predictors of poor sleep quality in dialysis patients was having history of coronary artery disease. Thus, our findings were in accord with previous findings.
Our study has some limitations that deserve mention. First, as our study is cross-sectional, cause and effect relationship cannot be suggested. Second, we make the measurements for once and temporal relationships may differ. Third, our findings only involved HD patients and these findings cannot be generalized to other patients with less severe kidney disease or peritoneal dialysis patients. Fourth, interdialytic changes in acid–base status were not evaluated. Finally, as mentioned above, polysomnography and HRV analysis was not performed.
In conclusion, we suggest that, metabolic acidosis and bicarbonate levels were independently related with sleep quality in HD patients. On the other hand we did not find any association between metabolic acidosis and bicarbonate levels with cognitive function and depression. Studies are needed to highlight underlying mechanisms regarding metabolic acidosis and bicarbonate and sleep problems.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Libório AB, Daher EF, de Castro MC. Characterization of acid–base status in maintenance hemodialysis: Physicochemical approach. J Artif Organs. 2008;11:156–159
- Kraut JA, Kurtz I. Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978–993
- K/DOQI Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201
- Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70–78
- Kalantar-Zadeh K, Mehrotra R, Fouque D, Kopple JD. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17:455–465
- Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004:44:661–671
- Driver TH, Shlipak MG, Katz R, et al. Low serum bicarbonate and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2014;64:534–541
- Goldenstein L, Driver TH, F Fried L, et al. Serum bicarbonate concentrations and kidney disease progression in community-living elders: The health, aging, and body composition (Health ABC) Study. Am J Kidney Dis. 2014;64:542–549
- Afsar B, Kirkpantur A. Are there any seasonal changes of cognitive impairment, depression, sleep disorders and quality of life in hemodialysis patients? Gen Hosp Psychiat. 2013;35:28–32
- Chan R, Brooks R, Erlich J, Chow J, Suranyi M. The effects of kidney-disease-related loss on long-termdialysis patients' depression and quality of life: Positive affect as a mediator. Clin J Am Soc Nephrol. 2009;4:160–167
- Molloy DW, Standish TI. A guide to the standardized mini-mental state examination. Int Psychogeriatr. 1997;9:87–94
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–571
- Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193–213
- Sanner BM, Tepel M, Esser M, et al. Sleep-related breathing disorders impair quality of life in hemodialysis recipients. Nephrol Dial Transplant. 2002;17:1260–1265
- Tada T, Kusano KF, Ogawa A, et al. The predictors of central and obstructive sleep apnoea in hemodialysis patients. Nephrol Dial Transplant. 2007;22:1190–1197
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107
- Sharp JT, Druz WS, D’Souza V, Diamond E. Effect of metabolic acidosis upon sleep apnea. Chest. 1985;87:619–624
- Fein AM, Niederman MS, Imbriano L, Rosen H. Reversal of sleep apnea in uremia by dialysis. Arch Intern Med. 1987;147:1355–1356
- Chan CT, Hanly P, Gabor J, Picton P, Pierratos A, Floras JS. Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int. 2004;65:661–665
- Roumelioti ME, Ranpuria R, Hall M, et al. Abnormal nocturnal heart rate variability response among chronic kidney disease and dialysis patients during wakefulness and sleep. Nephrol Dial Transplant. 2010;25:3733–3741
- Jung HH, Han H, Lee JH. Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am J Kidney Dis. 2005;45:875–882
- Unruh ML, Hartunian MG, Chapman MM, Jaber BL. Sleep quality and clinical correlates in patients on maintenance dialysis. Clin Nephrol. 2003;59:280–288

