Abstract
Introduction: Left ventricular hypertrophy (LVH) is a significant risk factor for cardiovascular complications in hemodialysis (HD) patients. Hypervolemia has been accepted as an independent risk factor for progressive LVH in HD patients. Additionally, high FGF23 levels have been a significant predictor of cardiovascular mortality and morbidity in chronic kidney disease and HD patients. The aim of our study is to investigate the correlation among LVH, interdialytic volume increase and FGF-23 in the patients on a chronic hemodialysis program. Design and methods: A total of 97 chronic hemodialysis patients (64.43 ± 11.28 years old, M/F:47/50) were included in the study. Human FGF-23 ELISA kit was used for FGF-23 analysis of predialysis blood samples. Echocardiographic evaluation was performed in all of the patients after dialysis. Left Ventricular Mass Index (LVMI) was calculated by using the Devereux Formula. We collected the following data: LVMI, FGF-23 levels, interdialytic fluid gain, blood pressure changes, and the other biochemical and clinical parameters. Results: Mean LVMI of the patients was 184.41 ± 48.62 g/m2. LVMI of the patients with daily urine output >250 mL was found significantly lower. Statistically significant positive correlation was found between predialysis systolic blood pressure, predialysis diastolic blood pressure, predialysis mean arterial blood pressure and LVMI measurements (p < 0.01). Mean interdialytic volume excess was correlated with LVMI measurements of the patients (r = 0.459; p < 0.01). Increased FGF-23 levels (159.79 ± 134.99 ng/L) predicted increased LVMI measurements of the patients (r = 0.322; p < 0.01). In addition, FGF-23 levels were also increased as the interdialytic fluid volume increased (r = 0.326; p < 0.05). A positive correlation was also found between FGF-23 levels and interventricular septum thickness (r = 0.238; p < 0.05). Predialysis mean arterial blood pressure, predialysis volume overload and presence of diabetes were determined to be independent risk factors on LVMI on multivariate regression analysis. Conclusion: Our study showed that interdialytic volume overload increased both LVMI and FGF-23 values. We can consider that interdialytic volume control exerts positive effects on increased FGF-23 levels which predict the negative cardiovascular outcomes.
Introduction
Cardiovascular problems are the most frequent complications during chronic hemodialysis (HD). Seventy-five percent of patients who start HD have left ventricular hypertrophy (LVH).Citation1,Citation2 LVH has been significantly associated with the longer HD duration.Citation3
Associated risk factors of progressive LVH on HD patients include age, gender, race, body mass index, diabetes, hypertension and hypervolemia.Citation4,Citation5 Hypervolemia has been accepted as an independent risk factor for all-cause and cardiovascular mortality in HD patients.Citation6
Recently, high FGF23 levels have been a significant predictor of cardiovascular mortality and morbidity in chronic kidney disease and HD patients.Citation7,Citation8
In this cohort study of 97 patients on chronic HD, we investigated the frequency of LVH, the risk factors on left ventricular mass index (LVMI), and the correlations between FGF23 levels with hypervolemia and LVMI.
Materials and methods
Our study was approved by the Ethical Committee of Clinical Studies, University of Istanbul Aydin. From two hemodialysis centers occupied by Istanbul Ozel Erdem Hospital, 97 patients over 18 years of age who were under three times a week hemodialysis programme for more than six months were included. Written acknowledgements of all the patients were obtained.
Patients who were under the dialysis for less than six months, hemodynamically unstable during dialysis, under poor general condition and patients with functional capacity class NYHA (New York Heart Association) III–IV or signs of acute coronary syndrome were excluded from the study.
For all the patients, Fresenius FX CorDiax High-Flux dialyzers (FX Class 60, FX Class 80 and FX Class 100, Fresenius SE & Co. KGaA, Hesse, Germany) appropriate for body surface areas were used. The dialysate was composed of Na: 140 mmol/L, K: 1–2 mmol/L. The patients were not allowed to eat during the four hours-dialysis period. Blood samples were taken for FGF-23 analysis and the other biochemical parameters from all of the patients before dialysis. FGF-23 test analysis was performed in Biochemistry laboratory of Gulhane Military Medical Academy Haydarpasa Training Hospital. Blood samples of the patients were taken for FGF-23 measurement and then plasma samples were centrifuged and frozen at −70 °C until the analysis. Serum FGF-23 levels were measured by Human FGF-23 ELISA kits (the range of detection: 5–1500 pg/mL, sensitivity: 2.49 pg/mL, intraassay CV <10% and interassay CV: <10%, Eastbiopharm, Hangzhou Eastbiopharm Co. Ltd., Hangzhou, China).
Twenty-four hour urine outputs of the patients were measured. Comparing with the dry weights, interdialytic volume increases were calculated and ultrafiltration was planned according to these amounts. Predialysis and postdialysis blood pressure values were also recorded.
The patients were evaluated by using echocardiography after dialysis. The measurements were performed in the lateral decubitus position by using a Vivid S 5 (Wauwatosa, WI) device and 3 S (mHz) adult probe. Left ventricular functions were assessed by M mode and two-dimensional images in parasternal long-axis, short-axis and apical 2–4 chamber images. Left ventricular internal end-systolic diameter (LVESD), left ventricular internal end-diastolic diameter (LVEDD), end-diastolic interventricular septum thickness (IVSTd), end-diastolic posterior wall thickness (PWTd), left and right atrial diameters (LAD and RAD) were measured. Left ventricular ejection fraction was calculated using modified Simpson’s rule.Citation9 Left ventricular mass index (LVMI) was calculated by using Devereux formula.Citation10
Left ventricular mass index (LVMI: g/m2) was obtained by dividing left ventricular muscle by body surface area. Body surface area (BSA) was obtained by entering height and body measurements by using DuBois formula.
The patients with LVMI ≥125 g/m2 in the males and the patients with LVMI ≥110 g/m2 in the females were considered to be hypertrophic.Citation11 Under the influence of the data about the correlations between left ventricular hypertrophy and FGF-23 levels, volume status, 24-hour urine outputs; we searched for other clinical and biochemical parameters.
Statistical evaluation
NCSS (Number Cruncher Statistical System) 2007&PASS (Power Analysis and Sample Size) 2008 Statistical Software (Kaysville, UT) program was used for the statistical analysis. Study variables were shown as mean, standard deviation, median, minimum, maximum, frequency and ratio in the table and the text. During the statistical analysis of the data obtained from the study, Student T test was used for the intergroup comparisons of variables with normal distribution; Kruskal–Wallis test was used for the comparisons of more than three groups; Mann–Whitney U-test was used for determination of the group causing difference and for the dual comparisons of groups. Pearson’s and Spearman’s correlation analysis was used for evaluation of the correlations between the variables. In determination of the risk factors effective on LVMI, multivariate analyses were performed by using linear regression analysis. Fisher–Freeman Halton test was used for the comparison of qualitative data. The results were evaluated in 95% confidence interval and at a significance level of p < 0.05.
Results
A total of 97 adult patients with a mean age of 64.43 ± 11.28 years from two hemodialysis centers were included in the study. The frequency for dialysis was three times per week, the mean duration of dialysis was 65.28 ± 49.26 months and distribution of gender was M/F: 47/50. Among the patients, the most common cause of renal failure was diabetes (n = 40). Hypertension was the second leading cause (n = 37). Kt/V level, which is parameter of dialysis adequacy, of all cases was 1.62 ± 0.25. General characteristics of the patients were summarized in .
Table 1. General characteristics of patients.
When the urine outputs of the patients were evaluated; the daily urine output was found to be less than 250 mL in 46 patients (47.4%) whereas it was more than 250 mL in 51 patients (52.6%). Mean weights of the patients in predialysis and postdialysis periods were 73.86 ± 15.99 and 70.97 ± 16.09 kg. respectively; hence the average amount of ultrafiltration was 2.68 ± 0.90 liter.
Predialysis systolic, diastolic and mean blood pressures (MAP) were assessed as 136.44 ± 14.29 (100–170) mmHg, 78.40 ± 9.56 (60–100) mmHg and 97.74 ± 10.05 (73.3–123.3) mmHg; respectively. After the dialysis, systolic and diastolic blood pressures were measured to be 120.05 ± 15.30 (85–140) mmHg and 73.45 ± 8.87 (50–90) mmHg, respectively.
Left Ventricular Mass Index (LVMI) of the patients varied between 105.66 and 353.73 and mean LVMI was determined as 184.41 ± 48.62. LVMI was increased in 93.6% (44) of the male patients and in 98% (49) of the female patients.
When the factors effective on the left ventricular hypertrophy were investigated, the correlation between residual renal functions and left ventricular mass index was observed to be highly significant (p < 0.01). LVMI of the patients with daily urine output >250 mL was found to be significantly lower. In case of renal failure, it was observed that neither hypertension nor uncontrolled diabetes (HbA1C >7) was associated with LVMI values. Also, no correlation was determined between the duration of dialysis, lipid levels and the levels of parathyroid hormone (iPTH) of the patients and LVMI ().
Table 2. Assessments regarding LVMI measurements.
Mean ejection fraction (EF) of the patients was calculated as 55.52 ± 8.97. Negative correlation between EF measurements and LVMI measurements at a level of 28.7% (the value of EF decreased as the value of LVMI increased) was found to be statistically significant (r = −0.287; p < 0.01).
Positive correlation between predialysis systolic, diastolic and mean blood pressure measurements and LVMI measurements was highly significant (r = 0.472; p = 0.001; r = 0.333; p = 0.001; r = 0.411; p = 0.001; respectively).
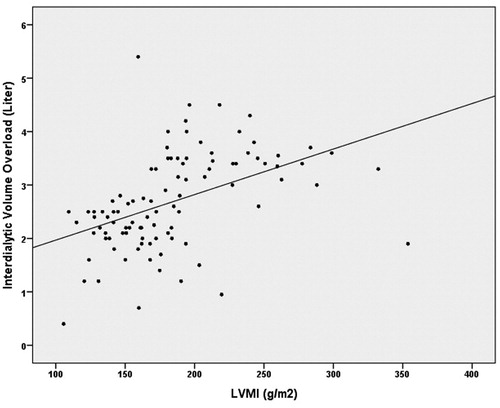
Also, the correlation between mean interdialytic volume overload and LVMI measurements of the patients at a level of 45.9% was found to be highly statistically significant (r = 0.459; p < 0.01) (). It was observed that LVMI was also significantly higher in the patients who had more predialysis volume overload.
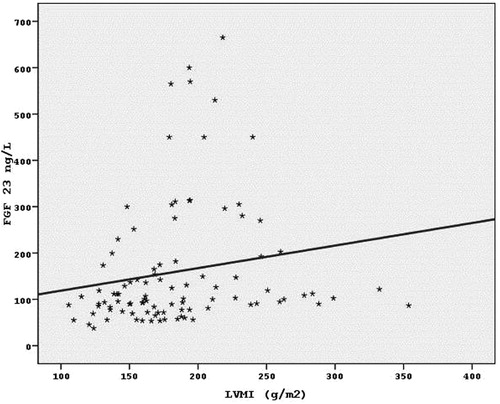
The mean FGF-23 levels of the patients were determined to be 159.79 ± 134.99 ng/L. Positive correlation between FGF-23 measurements and LVMI measurements (the value of FGF-23 also increased as the value of LVMI increased) at a level of 32.2% was found to be highly statistically significant (r = 0.322; p < 0.01) ().
As the connection between the FGF 23 levels and clinical and biochemical data of the patients was examined, it was observed that if the dialysis duration was prolonged, FGF 23 levels were increased (r = 0.252; p < 0.05). The positive relation between the plasma phosphorus and FGF levels was also determined (r = 0.220; p < 0.05). Meanwhile, CaxP levels increased with FGF 23 levels accordingly (r = 0.258; p < 0.05). No significant correlation was determined between the plasma FGF-23 levels and age, gender, calcium levels, iPTH levels and the presence of diabetes and/or hypertension (p > 0.05).
From the echocardiographic parameters; only IVS thickness was found to be significantly correlated with FGF-23 levels. IVS thickness increased with FGF-23 levels accordingly (r = 0.238; p < 0.05) ().
Table 3. Assessments of FGF-23 levels and data related to the patients.
When the correlations between FGF-23 levels and changes in blood pressures were investigated, it was observed that FGF-23 levels also increased statistically, significantly as predialysis systolic blood pressure increased (r = 0.211; p < 0.05). No significant correlation was determined between FGF-23 levels and diastolic blood pressure and MAB measurements (p > 0.05).
A positive statistically significant correlation was determined between FGF-23 levels and predialysis volume overload of the patients calculated according to the dry weights (r = 0.326; p < 0.05). Significantly, higher FGF-23 levels were observed as the interdialytic volume overload increased ().
When we evaluated the risk factors effective on the increase of LVMI (mean arterial pressure, interdialytic volume overload, presence of diabetes, 24-hour urine output, FGF-23 levels, serum phosphorus levels, CaXP and iPTH) by using multivariate regression analysis; the effects of predialysis mean arterial pressure, interdialytic volume overload (volume overload) and DM on LVMI were found to be statistically significant (p < 0.05). The effects of other variables were found to be non-significant (p > 0.05).
In our study, mean arterial pressure, interdialytic volume overload and presence of diabetes in the chronic hemodialysis patients were determined to be independent risk factors effective on LVMI increase ().
Table 4. Linear regression analysis of risk factors effective on LVMI.
Discussion
LVH is a frequently encountered morbidity in chronic HD patients. Although 75% of patients who start HD have LVH, unfortunately the frequency significantly increases by the HD duration.Citation1,Citation3,Citation12 In the Charytan’s review, LVH has been reported in 68–89% of end-stage renal disease.Citation13 Higher LVH percentage in our study population was correlated with the longer mean HD duration.
One of the promising results of our study is the definitive relation between hypervolemia and LVH. LVH frequency significantly increases with the increasing interdialytic volume load of the patients. Charytan also reviewed that volume control with more frequent HD programs may regress LVH.Citation13
LVMI has been significantly great in predialysis patients with a 24-hour urine output of <250 mL. In Davenport’s seminar, the progressive loss of residual renal function in dialysis patients has been associated with increased mortality.Citation14 Declining residual functions result with decreased urine output which produces a steady hypervolemia state in the patients.
Concepts of hypervolemia and dry weight have long been an investigated and updated issue in the daily practice of dialysis.Citation15–19 One of the consequences of hypervolemia is volume-related hypertension. In our study, LVMI was increased by the high blood pressure levels at the beginning of dialysis
Associated dependent risk factors of LVH include mean arterial pressure at the beginning of dialysis, interdialytic volume load and diabetes all of which are compatible with the current literature.Citation2,Citation3,Citation5
We also investigated the relationship between recently discovered FGF23 concentrations with cardiovascular risk factors, i.e. hypervolemia and LVH.
FGF23 as a novel marker in CKD and HD patients is implicated in phosphate and vitamin D metabolism. FGF23 is also defined as major phosphaturic factor.Citation20 FGF23 concentration progressively increases in CKD patients. Increased FGF23 levels is related with accelerated atherosclerosis and endothelial dysfunction, and accepted as a predictor of mortality.Citation7,Citation8
Lutsey et al.Citation21 reported that FGF23 was positively associated with cardiovascular risk at >40 pg/mL (=ng/L). In our study, the mean FGF23 of our participants was 159.79 ± 134.99 ng/L.
Agarwal et al. reported that among 887 participants with coronary artery disease, FGF23 was only associated with concentric hypertrophy among individuals with diminished kidney function (eGFR < 60 mL/minute) and suggested that FGF23 has major effects on cardiovascular structure and function among patients with CKD.Citation22
Diniz, also, observed that increased levels of FGF23 in CKD patients are associated with cardiovascular mortality risk.Citation23
In the study by Sany et al., FGF23 was identified as a weakly associated factor with LVMI in 90 consecutive long-term HD patients without cardiac symptoms.Citation24
In our study, the serum FGF23 levels were significantly correlated with both LVMI and interventricular septum thickness. Our analysis revealed that FGF23 showed a strong association with the longer duration of HD, serum phosphate levels and CaXP. In this study comprising a cohort of patients with chronic HD, we identified that FGF23 was significantly correlated with predialysis systolic BP and increased interdialytic volume overload.
FGF23 has been implicated in the bone and mineral metabolism and increased levels of FGF23 in HD patients has been accepted as an independent cardiovascular morbidity and mortality risk factor. Our study is the first in the current literature that investigated the significant relationship between increased FGF23 levels and hypervolemia in HD patients. Therefore, FGF23 may be accepted as a volume marker.
Conclusion
Hypervolemia in dialysis patients not only causes left ventricular hypertrophy but also increases FGF-23, which is considered to be one of the most powerful predictors of cardiovascular mortality. Efficient interdialytic volume control can decrease FGF-23 levels as well as regressing left ventricular hypertrophy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–192
- Foley RN, Parfrey PS. Risk factors for cardiac morbidity and mortality in dialysis patients. Curr Opin Nephrol Hypertens. 1994;3(6):608–614
- Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5(5):805–813
- Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS. Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol. 1994;4(7):1486–1490
- McMahon LP, Parfrey PS: Cardiovascular aspects of chronic kidney disease. In: Brenner BM, ed. Brenner and Rector’s The Kidney. 8th ed. Philadelphia: Saunders Elsevier; 2008:1697–1727
- Tsai YC, Chiu YW, Tsai JC, et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol. 2015;10(1):39–46
- Russo D, Battaglia Y. Clinical significance of FGF-23 in patients with CKD. Int J Nephrol. 2011;2011:364890
- Komaba H. CKD-MBD (Chronic Kidney Disease-Mineral and Bone Disorder). Role of FGF23 Klothoaxis in CKD-MBD. Clin Calcium. 2010;20(7):1028–1036
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458
- Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9(2 Pt 2):II19–26
- Laddha M, Sachdeva V, Diggikar PM, Satpathy PK, Kakrani AL. Echocardiographic assessment of cardiac dysfunction in patients of end stage renal disease on hemodialysis. J Assoc Physicians India. 2014;62(1):28–32.
- Charytan D. Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23:578–585
- Davenport A. Will incremental hemodialysis preserve residual function and improve patient survival? Semin Dial. 2015;28(1):16–19
- Leunissen KML. Fluid status in hemodialysed patients. Nephrol Dial Transplant. 1995;10:153–155
- Charra B. ‘Dry weight’ in dialysis: the history of a concept. Nephrol Dial Transplant. 1998;13:1882–1885
- Luik AJ, Charra B, Katzarski K, et al. Blood pressure control and hemodynamic changes in patients on long time dialysis treatment. Blood Purif. 1998;16:197–209
- Wizemann V, Schilling M. Dilemma of assessing volume state: The use and limitations of a clinical score. Nephrol Dial Transplant. 1995;10:2114–2117
- Weiner DE, Brunelli SM, Hunt A, et al. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of us dialysis providers. Am J Kidney Dis. 2014;64(5):685–695
- Imanishi Y, Kobayashi K, Kawata T, Inaba M, Nishizawa Y. Regulatory mechanisms of circulating fibroblast growth factor 23 in parathyroid diseases. Ther Apher Dial. 2007;111:S32–37
- Lutsey PL, Alonso A, Selvin E, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: The atherosclerosis risk in communities study. J Am Heart Assoc. 2014;3(3):e000936
- Agarwal I, Ide N, Ix JH, et al. Fibroblast growth factor-23 and cardiac structure and function. J Am Heart Assoc. 2014;3:e000584
- Diniz H, Frazão JM. The role of fibroblast growth factor 23 in chronic kidney disease-mineral and bone disorder. Nefrologia. 2013;33(6):835–844
- Sany D, Elsawy AE, Aziz A, et al. The value of serum FGF-23 as a cardiovascular marker in HD patients. Saudi J Kidney Dis Transpl. 2014;25(1):44–52