Abstract
Background: The predictive value of heart rate variability (HRV) in peritoneal dialysis (PD) has never been tested. Methods: In this study, the associations between HRV measures and the mortality in 81 PD patients were analyzed. HRV was measured by using 5-min recordings of a stationary system by a standardized method. Both time domain and frequency domain parameters were analyzed. Results: During a follow-up period of 43.78 ± 14.77 months, 25 patients died, four patients were transferred to hemodialysis. Of the 81 patients, the time domain parameters, such as the standard deviation of differences between adjacent normal sinus to normal sinus (NN) intervals (SDSD) and the square root of the mean of the squared differences between adjacent normal NN intervals (RMSSD), were higher; the frequency domain parameters, such as the ratio of low-frequency power to high-frequency power (LF/HF) and the normalized LF, were lower, and the normalized HF was higher in the non-survived group as compared with the survived group. A Cox proportional hazards model analysis revealed that, of the HRV measures, decrease of the normalized LF, LF/HF and increase of rMSSD, SDSD, normalized HF had significant predictive value for mortality. After adjustment for other univariate predictors including age, urine volume, renal Kt/V, high-sensitivity C-reactive protein (hs-CRP), the predictive value of decreased LF/HF remained significant. Kaplan–Meier survival analysis showed mortality rate was much higher in patients with a low LF/HF (median value of 1.56). Conclusion: The decreases of LF/HF which reflects impaired sympathetic nerve regulation is an independent predictor of mortality in PD patients.
Introduction
The mortality of patients with end-stage renal disease (ESRD) on chronic peritoneal dialysis remains high despite significant advances in technologies in the past decades.Citation1–3 Factors related to mortality include age, serum albumin level, protein–energy wasting (PEW), high-sensitivity C-reactive protein (hs-CRP) level, cardiovascular disease, peritonitis, comorbidity, depression, volume status, residual kidney function (RRF), etc.,Citation4–8 in which cardiovascular death accounts for more than half of the death.
Heart rate variability (HRV), which is defined as “the amount of fluctuations from the mean heart rate”, is primarily controlled by the continuous interplay of the autonomic nervous system, and it is used as a useful noninvasive tool to assess the autonomic function. Reduced HRV has prognostic value in various populations,Citation9–12 and it is reported that reduced HRV predicted an increased risk of mortality in chronic hemodialysis (HD) patients and non-dialysis chronic kidney disease (CKD) patients.Citation13,Citation14 However, the prognostic effect of HRV on peritoneal dialysis patients remains unclear.
Therefore, in the present study, we tried to investigate the predictive effect of HRV analysis parameters on mortality of continuous ambulatory peritoneal dialysis (CAPD) patients.
Subjects and methods
Study populations
This is a prospective observational study. In December 2007, CAPD patients from our peritoneal dialysis (PD) unit were enrolled. The exclusion criteria were: (a) dialysis duration of less than 3 months, (b) unwillingness to participate in the study, (c) a cardiac event that occurred less than 3 months before the study, (d) a history of hemodynamically significant valvular or congestive heart failure, (e) atrial fibrillation or flutter and (f) high grade heart block or a permanent pacemaker.
Analysis of HRV
A dedicated doctor was in charge of all HRV measurements with standardized method. Briefly, all patients were studied in a quiet, comfortable room from 8 am to 10 am. Before recording, we asked all patients to breathe at normal frequency and the respiratory rate was recorded. The patients were then lying in supine position for at least 10 min before starting a baseline. The patients underwent ambulatory ECG recording for 5 min. All recordings were analyzed using ECG Explorer 500 A pc electrocardiograph (Multiparameter Analysis and Review System).
An automatic analysis was made to classify QRS morphology, to distinguish normal and non-normal QRS complexes and to identify normal sinus rhythm, to exclude ectopic beats and arrhythmias that would interfere with HRV analysis. The following time domain analyses of normal (NN) variability were calculated: the standard deviation of all normal NN intervals (SDNN, ms); the standard deviation of differences between adjacent NN intervals (SDSD, ms); the square root of the mean of the squared differences between adjacent normal NN intervals (RMSSD, ms); the percentage of differences between adjacent normal NN intervals that were >50 ms computed (pNN50, %).
Frequency domain measures of R–R variability were assessed with power spectral analysis of ECG recordings by a nonparametric (fast Fourier transformation) method for the following frequency bands: the low-frequency energy in the power spectrum 0.0400–0.1500 Hz (LF, ms2); the high-frequency energy in the power spectrum 0.1500–0.400 Hz (HF, ms2); the total power spectrum of 0.0033–1.7070 Hz (TP, ms2) and the ratio of low- to high-frequency power (LF/HF). The LF and HF bands were expressed in normalized units (LF normalized units = LF/[TP − VLF]; HF normalized units = HF/[TP − VLF]).Citation15
Bioimpedance analysis
Multiple-frequency bioelectrical impedance analysis (BIA) was performed with the Hydra analyzer (Xitron Technologies, San Diego, CA) after patients emptied their dialysis solutions. The procedure to perform this measurement was described in detail elsewhere. From this measurement, the estimation of extracellular water (ECW), intracellular water (ICW) and total body water (TBW) could be acquired.
Measurement of BP, biochemistry and dialysis adequacy
A dedicated renal nurse was in charge of all blood pressure measurements, and the mercury sphygmomanometer used was calibrated regularly. All measurements were performed in a quiet room. Brachial blood pressure was measured three times in the sitting position after patients had rested >10 min. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were averaged from values of the three measurements. Serum albumin was determined by the bromocresol green method. Other biochemical indices, such as blood urea, serum creatinine, were determined using standard methods. Residual renal function (RRF) was measured as glomerular filtration rate (GFR) using the mean of urea and creatinine clearances. Total fluid removal (TFR) was the sum of daily urine and ultrafiltration (UF). Small solute removal was determined by measurement of total (peritoneal dialysis and renal) weekly urea Kt/V using standard methods. The contributions to total Kt/V (tKt/V) by peritoneal dialysis (pKt/V) and RRF (rKt/V) were estimated separately. The volume of urea distribution (V) was derived using Watson's formula.
Subjective global assessment (SGA)
SGA scores were used to assess patient’s nutritional status. SGA comprises six subjective assessments, three based on the patient’s history of weight loss, presence of anorexia and vomiting, and three based on the examiners’ grading of muscle wasting, presence of edema and loss of subcutaneous fat. On the basis of these assessments, patients’ nutritional status was graded as “A”, “B” or “C”, reflecting normal nutrition, mild protein–energy wasting (PEW) and moderate or severe PEW, respectively. In this study, no patient was characterized as Grade “C”.
Follow-up
Patients were followed until the end of point (death, transfer to HD or transplant) or until 31 December 2011. Survival times were recorded from the day of the time of the 5-min electrocardiogram recording until death or censoring. The event of this study was death. Survival times were considered to be censored at the date of a kidney transplant, transfer to HD or at the end of the follow-up period on 31 December 2011. All the baseline data in the present analysis also referred to the time when the 5-min electrocardiogram recording was done.
Statistical analysis
Statistical analysis was performed using SPSS for Windows software, version 12.0 (SPSS Inc., Chicago, IL). Measures of HRV were analyzed after logarithmic transformation. All data were expressed as mean ± SD for continuous variables or percentages for categorical variables. To compare different groups, unpaired Student's t-test was used for continuous variables as appropriate, the Chi-square test was used for categorical variables. Survival analysis was performed using a Cox proportional hazards model. Variables that achieved statistical significance in the univariate analysis were subsequently included in a multivariate analysis using a stepwise Cox regression procedure. Kaplan–Meier cumulative survival curves were constructed and compared using the log rank test. All tests were two-sided, a p-value below 0.05 was considered significant.
Results
Baseline clinical data and outcomes
Eighty-one patients who met the inclusion criteria were included in the present study. The demographic characteristic of the study population is shown in . During the 4-year follow-up period (mean 43.78 ± 14.77 months), 25 patients died (two sudden death, two acute myocardial infarctions, six fatal strokes, three malignant neoplasms, four multiple organ failure and eight severe infection), four patients were transferred to HD (three because of peritonitis and one because of economical reason).
Table 1. Demographic characteristics of the study population.
The prognostic factors for mortality
Baseline parameters of patients grouped by survival status are shown in . Compared with the patients who survived during follow-up, those who died were older and had lower levels of urine volume, DBP, serum potassium, ICW, GFR and rKt/V. There were no significant differences between the two groups on gender, body mass index (BMI), PD duration, diabetes, SBP, creatinine, blood urea nitrogen (BUN), PEW, hemoglobin, albumin, ECW, TBW, hs-CRP, parathyroid hormone (PTH), high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides, pKt/V, tKt/V, UF, TFR, use of beta-blocker and angiotensin-converting enzyme inhibitors (ACEI).
Table 2. Clinical features of patients grouped by survival status.
The analysis of HRV measures in peritoneal dialysis patients grouped by survival status is shown in . In the group of non-survival, rMSSD and SDSD were higher, the normalized LF and LF/HF were lower and the normalized HF was higher as compared with the survival group. There were no significant differences in other indices of HRV between the two groups.
Table 3. HRV measures of patients grouped by survival status.
The univariate analysis of the relative risk for mortality is shown in and . Elder, lower urine volume, rKt/V, serum potassium, ICW and higher hs-CRP are all risks for high mortality. Among HRV measures, a lower normalized LF, LF/HF and a higher rMSSD, SDSD were related to high mortality.
Table 4. Univariate Cox regression analysis of clinical characteristics related to death.
Table 5. Univariate Cox regression analysis of heart rate variability measures in relation to death.
Multivariate Cox regression analysis showed that urine volume, hs-CRP and LF/HF were three independent prognostic factors for death during the follow-up (). shows the comparison of survival rate of patients with different LF/HF values (grouped by median of LF/HF, LF/HF >1.56 vs. ≤1.56). The 4-year mortality rate for the patients who had an LF/HF of >1.56 or ≤1.56 was 17.5% or 43.9%, respectively. Lower LF/HF (≤1.56) was a strong predictor for high mortality. shows that patients with urine volume of >100 mL per day had significantly better survival. shows that the inflamed patients who were defined as hs-CRP values higher than 3 mg/L had significantly higher mortality as compared to the non-inflamed patients.
Figure 1. Kaplan–Meier survival curves for death in patients dichotomized by the median values of LF/HF. LF, low frequency; HF, high frequency.
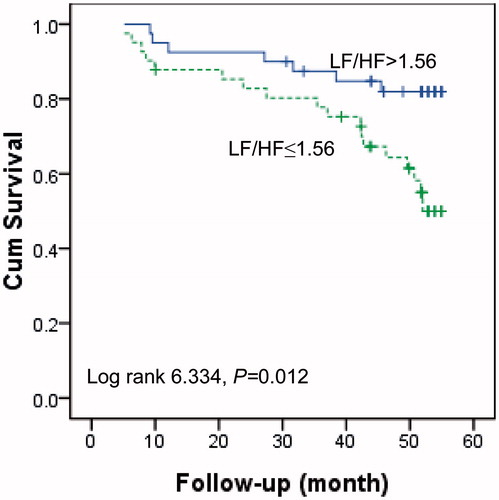
Figure 2. Kaplan–Meier survival curves for cardiac death in patients with a urine volume of >100 versus ≤100 mL/L.
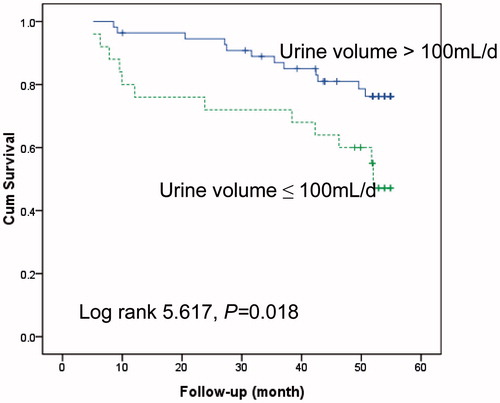
Figure 3. Kaplan–Meier survival curves for death in patients with a hs-CRP >3 mg/L versus ≤3 mg/L. hs-CRP, high-sensitivity C-reactive protein.
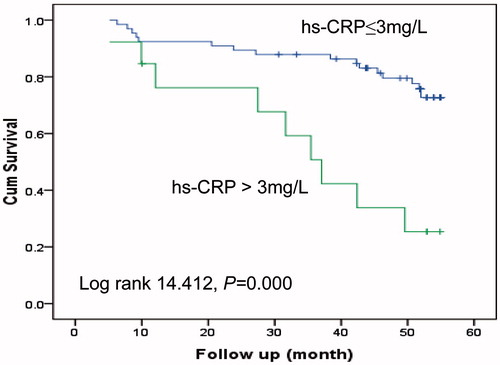
Table 6. Multivariate Cox regression analysis of clinical and HRV measures in relation to death.
Discussion
In this prospective study, we examined the prognostic value of 5-min HRV measures in 81 chronic peritoneal dialysis patients with a follow-up period of 43.78 ± 14.77 months. A mortality predictive effect of HRV indictors especially LF/HF values was observed by the present study even with adjustment of other confounding factors. To our knowledge this is the first report investigating the prognostic value of HRV in PD patients.
The abnormalities of HRV in patients with renal impairment have been documented by pervious study. It has been found that chronic PD and HD patients had reduced HRV as compared to the healthy controls.Citation16,Citation17 Furuland et al. showed that CKD patients not yet on dialysis had a reduced HRV (both time domain and frequency domain) as compared with a reference group without impaired renal function.Citation18 Chandra et al. found that lower HRV (frequency domain) was independently associated with higher risk of CVD, the composite of CVD/death and ESRD in non-dialysis CKD patients.Citation14 Fukuta et al. found that HRV is reduced in chronic HD patients compared with healthy individuals, further, those patients with greatest reduction in this variable also have the greatest risk for cardiac death.Citation13 Hayano et al. showed that HRV has an independent prognostic value in chronic HD patients and identifies an increased risk for all-cause and sudden death,Citation19 and Oikawa et al. reported that decreased HRV (SDNN) on 24-h ambulatory ECG was an independent predictor of all-death and cardiovascular death in chronic HD patients.Citation20 However, this predictive value of HRV in PD patients has not been elucidated previously. Thus, our study added valuable evidence in this area.
In our study, a link between decreased LF/HF and high mortality was discovered, which is consistent with a recent study showing that the low LF/HF has a prognostic value of CVD and ESRD in non-dialysis CKD patients,Citation14 and this observation has been confirmed by Furuland et al.;Citation18 a decrease in LF/HF can significantly predict cardiac death in CKD patients. Brotman et al.Citation21 studied 13,241 adults (45–64-years old) followed for a median of 16 years in the Atherosclerosis Risk in Communities study and underwent 2-min ECG monitoring between follow-up periods to assess prognostic value of HRV. Multivariate Cox proportional hazard model revealed that low LF/HF and other HRV parameters could predict higher hospitalization and development of kidney disease, and the reduced LF/HF could predict cardiac death in chronic HD patients.Citation13 With these results it is evident that a reduced LF/HF has an independent prognostic value in communities, CKD and chronic HD patients and identifies patients with an increased risk for hospitalization, development of kidney disease, cardiac-death and all-cause death.
The potential mechanism to explain the relationship between reduced LF/HF and mortality remains unclear. As the LF/HF is considered by some investigators to mirror sympatho/vagal balance or to reflect sympathetic modulations, we speculated that this impaired autonomic nerve system in PD patients may result in inefficiency of the natural stress coping system and thus subject the patients to high mortality when comorbidity occurs. Further study is warranted in this field.
In line with previous studies, our study also showed that anuric status and hs-CRP were strong predictors of death in PD patients.Citation8,Citation22 It is interesting to note that our study found that the non-survival group had a lower ICW as compared to the survival group. ICW reflects water content in the body cell mass.Citation23 Changes in body protein mainly occur in the cellular compartment;Citation24 consequently, the alterations in body protein are generally accompanied by changes in ICW.Citation25 A decreased ICW may indicate the loss of intracellular proteins caused by protein–energy malnutrition, which is common in PD patients.
Our study found that rMSSD and SDSD were almost the same, since many studies showed that the time domain measures correlated closely with others,Citation15,Citation26 our observations seem basically in line with those studies.
We conclude that decrease of LF/HF which shows impaired sympathetic nerve modulation is an independent predictor of mortality in PD patients.
Declaration of interest
There are no financial conflict of interest to report.
References
- Cala S. Peritoneal dialysis in Croatia. Perit Dial Int. 2007;27:238–244
- Nakai S, Iseki K, Itami N, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther Apher Dial. 2012;16:11–53
- Buargub MA. 5-Year mortality in hemodialysis patients: A single center study in Tripoli. Saudi J Kidney Dis Transplant. 2008;19:268–273
- Han SH, Han DS. Nutrition in patients on peritoneal dialysis. Nat Rev Nephrol. 2012;8:163--175
- Munoz de Bustillo E, Borras F, Gomez-Roldan C, et al. Impact of peritonitis on long-term survival of peritoneal dialysis patients. Nefrologia. 2011;31:723–732
- Ver Halen N, Cukor D, Constantiner M, Kimmel PL. Depression and mortality in end-stage renal disease. Curr Psychiatry Rep. 2012;14:36–44
- Szeto CC, Wong TY, Leung CB, et al. Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int. 2000;58:400–407
- Rocco MV, Frankenfield DL, Prowant B, Frederick P, Flanigan MJ. Risk factors for early mortality in US peritoneal dialysis patients: Impact of residual renal function. Perit Dial Int. 2002;22:371–379
- Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262
- Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171
- Farrell TG, Bashir Y, Cripps T, et al. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Cardiol. 1991;18:687–697
- Tsuji H, Venditti FJ Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883
- Fukuta H, Hayano J, Ishihara S, et al. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic hemodialysis. Nephrol Dial Transplant. 2003;18:318–325
- Chandra P, Sands RL, Gillespie BW, et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant. 2012;27:700–709
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381
- Hathaway DK, Cashion AK, Milstead EJ, et al. Autonomic dysregulation in patients awaiting kidney transplantation. Am J Kidney Dis. 1998;32:221–229
- Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: An objective evaluation of autonomic nervous control in chronic renal failure. Nephron. 1987;45:202–206
- Furuland H, Linde T, Englund A, Wikstrom B. Heart rate variability is decreased in chronic kidney disease but may improve with hemoglobin normalization. J Nephrol. 2008;21:45–52
- Hayano J, Takahashi H, Toriyama T, et al. Prognostic value of heart rate variability during long-term follow-up in chronic hemodialysis patients with end-stage renal disease. Nephrol Dial Transplant. 1999;14:1480–1488
- Oikawa K, Ishihara R, Maeda T, et al. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol. 2009;131:370–377
- Brotman DJ, Bash LD, Qayyum R, et al. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol. 2010;21:1560–1570
- Zalunardo NY, Rose CL, Ma IW, Altmann P. Higher serum C-reactive protein predicts short and long-term outcomes in peritoneal dialysis-associated peritonitis. Kidney Int. 2007;71:687–692
- Earthman CP, Matthie JR, Reid PM, Harper IT, Ravussin E, Howell WH. A comparison of bioimpedance methods for detection of body cell mass change in HIV infection. J Appl Physiol. 2000;88:944–956
- James HM, Dabek JT, Chettle DR, et al. Whole body cellular and collagen nitrogen in healthy and wasted man. Clin Sci (Lond). 1984;67:73–82
- Beddoe AH, Streat SJ, Hill GL. Hydration of fat-free body in protein-depleted patients. Am J Physiol. 1985;249:E227–E233
- Berntson GG, Bigger JT Jr, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648

