Abstract
Aim: To investigate the nature of dyslipidemia and its diversity in patients with systemic AA amyloidosis. Methods: The reports of the kidney biopsies performed due to nephrotic proteinuria (>3.5 g/day/1.73 m2) with preserved renal function [glomerular filtration rate (GFR) >60 mL/min/1.73 m2] were reviewed. Clinical and laboratory data of the patients with systemic AA amyloidosis and primary glomerulonephritis (PG) were analyzed. Results: A total of 104 (systemic AA amyloidosis: 43, PG: 61) patients were included in the study. Proteinuria and GFR levels were similar in both the groups. Patients with systemic AA amyloidosis group had lower serum albumin (p = 0.002), lower hemoglobin levels (p = 0.001), higher platelet counts (p = 0.002) and higher C-reactive protein levels (p = 0.001) compared to patients in PG group. Although the frequency of dyslipidemia was similar in the groups (86.0 vs. 93.4%), patients with systemic amyloidosis had both lower values of LDL-C (4.56 ± 2.05 vs. 5.49 ± 2.23 mmol/L, p = 0.028) and HDL-C (1.19 ± 0.36 vs. 1.35 ± 0.39 mmol/L, p = 0.035). Serum lipid levels were correlated with serum total protein, albumin and proteinuria levels in PG group. However, in the systemic amyloidosis group, only one clear correlation between serum lipid and hemoglobin levels was estimated. A multivariate analysis demonstrated that LDL-C was independently associated with the etiology of nephrotic proteinuria, serum total protein, serum albumin (inversely) and hemoglobin levels. Conclusions: Although dyslipidemia is closely associated with serum total protein, albumin and proteinuria in patients with PG, there is no clear such association in patients with systemic amyloidosis. Correlation between serum lipid and hemoglobin levels in this group and other findings point out that probably complex mechanisms take place in dyslipidemia of nephrotic syndrome caused by systemic AA amyloidosis.
Introduction
Nearly a 150 years have passed since describing “amyloid” as a starch by Wirchow,Citation1 now we know lots of details about characteristics of the amyloidosis. Amyloidosis constitutes a heterogeneous group of diseases characterized by extracellular deposition of amyloid, a proteinaceous fibrillary material, in different tissues and organs.Citation2 Systemic serum amyloid A (AA) amyloidosis is the most frequent type of systemic amyloidosis around the world, predominantly in developing countries; with a reported prevalence varied between 0.5 and 4.5%.Citation3,Citation4 Generally, systemic AA amyloidosis is a complication of chronic inflammatory diseases with aggregation of AA amyloid fibrils, derived from serum AA protein which is an apolipoprotein constituent of high-density lipoprotein (HDL), synthesized in liver with stimulation of proinflammatory cytokines.Citation5,Citation6
Nephrological evaluation is important in systemic AA amyloidosis since renal involvement is frequent. In clinical practice, the most common sign of the systemic AA amyloidosis is usually a nephrotic-range proteinuria with or without impaired renal function.Citation7,Citation8 An important point is that pathophysiological mechanisms leading to nephrotic syndrome are different in systemic AA amyloidosis and primary glomerulonephritis. These pathophysiological differences also play role in lipid metabolism abnormalities. Hypercholesterolemia in nephrotic syndrome related to PG is associated to both increased synthesis and decreased catabolism.Citation9,Citation10 Although it is proposed that hypercholesterolemia in amyloidosis is less common than in other forms of the nephrotic syndrome, nature and effects of dyslipidemia in patients with systemic AA amyloidosis are not known and conclusive.Citation9 On the other hand, it is known that dyslipidemia is a well-known risk factor for cardiovascular disease and it may accelerate the progression of the kidney disease.Citation11,Citation12 Since renal involvement is a major cause of mortality in this population, dyslipidemia is an important aspect of the disease to control patients with systemic AA amyloidosis.
Dyslipidemia is one of the cardinal manifestations of the nephrotic syndrome and it is considered as diagnostic criteria.Citation9,Citation10 There are many reports evaluating dyslipidemia and its mechanisms. Most of them are based on studies evaluating lipid profiles of patients with primary glomerulonephritis-related etiologies. Low-density lipoprotein-cholesterol (LDL-C) and triglyceride (TG) levels are almost always elevated, with varied conclusions on changes in serum high-density lipoprotein-cholesterol (HDL-C) levels.Citation10–13 On the other hand, there is no conclusive data on changes in serum lipid profiles of patients with systemic AA amyloidosis. In the existing limited studies, it is proposed that increased synthesis and decreased catabolism of the serum lipids due to hypoalbuminemia and proteinuria cause dyslipidemia.Citation10,Citation13–15 This theory probably explains the lipid changes seen in primary diseases that are limited to the kidney. However, this may not be the particular mechanism responsible for dyslipidemia seen in systemic AA amyloidosis that has different pathophysiological mechanisms such as deposition of fibrillary protein.
In this report, we analyzed the lipid abnormalities and their outcomes in patients with systemic AA amyloidosis, in comparison with patients with nephrotic syndrome caused by primary glomerulonephritis-related etiologies.
Subjects and methods
Patient population
We retrospectively reviewed the native kidney biopsies of 615 adult patients which were performed between January 2003 and January 2011 in our institute. Patients with nephrotic range proteinuria (>3.5 g/day/1.73 m2) and preserved renal function (GFR ≥ 60 mL/min/1.73 m2, serum creatinine <114.92 µmol/L) were included in the study. Patients with history of diabetes, malignancy, chronic liver disease, adrenal failure, hypothyroidism and patients taking drugs that may alter lipid levels (statins, fibrates, niacin, glucocorticoids, thiazide diuretics, beta blockers, etc.) were excluded. A total of 43 patients with biopsy-proven (immunohistochemically) systemic AA amyloidosis and 61 primary glomerulonephritis with complete data were included in the study. Nephrotic range proteinuria was defined as urinary protein levels exceeding 3.5 g/1.73 m2/day in 24 h urine sample. The term “dyslipidemia” was defined as serum levels of total cholesterol (TC) ≥6.21 mmol/L (>240 mg/dL) or low-density lipoprotein cholesterol (LDL-C) >3.37 mmol/L (>130 mg/dL) or high-density lipoprotein cholesterol (HDL-C) <1.03 mmol/L (<40 mg/dL) for males and <1.30 mmol/L for females (<50 mg/dL) or serum triglyceride (TG) >1.69 mmol/L (>150 mg/dL) according to ATP III guideline. GFR at admission was estimated by the 4-variable Modification of Diet in Renal Disease Study (MDRD) equation for each participant. The study protocol was approved by the local ethics committee (Date and number; 09.02.2011, 2011-102).
Statistics
Distribution of the continuous variables was checked using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were presented as mean ± SD. Categorical variables were presented as percentages and numbers. Student’s t test (if normally distributed) or Mann–Whitney U test (if not normally distributed) was used to compare the continuous variables between the two groups. Categorical variables were compared with chi-square test or Fischer’s exact test.
Pearson’s correlation coefficients were computed to explore the relationship between serum lipid fractions and other measured continuous variables (e.g., albumin, proteinuria, etc.). In order to evaluate other potential associations of serum lipid levels, factors that had a plausible relationship with these measurements were introduced in a multiple linear regression model backward selection method.
The odd ratios (OR) and 95% confidence intervals (CI) were calculated. Statistical significance was considered at a 2-tailed value of p < 0.05. Statistical analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 18.0, SPSS Inc., Chicago, IL).
Results
One hundred and four patients (43 systemic AA amyloidosis and 61 PG) that met the inclusion criteria were analyzed in the study. Mean age of the patients was similar in both the groups. Male patients were more frequent in systemic AA amyloidosis group, while their body-mass indexes were lower (). Etiological factors of the systemic AA amyloidosis were familial Mediterranean fever (FMF) in 22 (51.3%), rheumatoid arthritis in 9 (20.9%), ankylosing spondylitis in 6 (13.9%) and bronchiectasis in 6 (13.9%) patients. Etiologies of primary glomerulonephritis (PG) were membranous nephropathy in 39 (63.9%), IgA glomerulonephritis in 7 (11.5%), membranoproliferative glomerulonephritis in 6 (9.8%), mesangioproliferative glomerulonephritis in 5 (8.2%) and focal segmental glomerulosclerosis in 4 (6.6%) patients.
Table 1. Laboratory characteristics of the patients with systemic AA amyloidosis and primary glomerulonephritis (PG) at the time of the diagnosis.
The systemic AA amyloidosis and PG groups had similar proteinuria, GFR and serum creatinine levels. Patients with systemic AA amyloidosis had lower serum total protein, albumin, hemoglobin, MCV (mean corpuscular volume), MPV (mean platelet volume) levels, but higher platelet counts and C-reactive protein (CRP) levels ().
Although the frequency of dyslipidemia was comparable between the groups (86.0% in systemic AA amyloidosis and 93.4% in patients with PG, p = 0.208), both serum levels of LDL-C and HDL-C were lower in systemic AA amyloidosis group (). Distributions of serum lipid abnormalities are detailed as two different classifications in . Overall, hyper-LDL-C abnormality was more frequent (85.2%), in patients with PG, compared to systemic AA amyloidosis patients (67.4%, p = 0.031).
Table 2. Distributions of serum lipid abnormalities in systemic AA amyloidosis and primary glomerulonephritis (PG).
All serum lipid parameters, but HDL-C, correlated with serum total protein, albumin and proteinuria levels in patients with primary glomerulonephritis, whereas all serum lipid levels, but TG, correlated with hemoglobin levels in systemic AA amyloidosis group (; and ).
Figure 1. Relation of serum LDL-C with serum total protein levels (A) in patients with primary glomerulonephritis etiologies and (B) in patients with systemic amyloidosis (LDL-C, mmol/L; serum total protein).
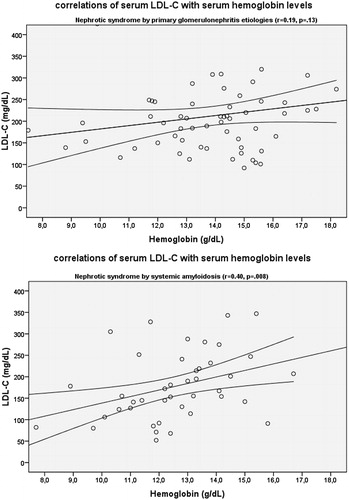
Figure 2. Relation of serum LDL-C with serum hemoglobin levels (A) in patients with primary glomerulonephritis etiologies and (B) in patients with systemic amyloidosis (LDL-C, mmol/L; hemoglobin g/L).
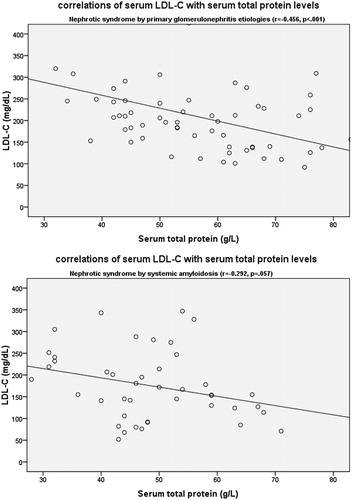
Table 3. Relationship of the serum lipid parameters with serum albumin, proteinuria and C-reactive protein in patients with systemic AA amyloidosis and primary glomerulonephritis (PG).
A multivariate analysis including the whole study group demonstrated that LDL-C was independently associated with the etiology of nephrotic syndrome (lower levels in patients with systemic AA amyloidosis compared to PG), serum total protein, albumin (inversely), and hemoglobin levels. HDL-C was related to gender (lower levels in male) and hemoglobin levels (). TG was related to serum total protein (inversely) and hemoglobin levels.
Table 4. A multivariate analysis of the overall patients with nephrotic proteinuria [both caused by systemic AA amyloidosis and primary glomerulonephritis (PG)].
Discussion
Majority of the patients with systemic AA amyloidosis and PG have dyslipidemia. Dyslipidemia is closely associated with serum total protein, albumin and proteinuria in patients with PG. On the other hand, there is no clear such association in patients with systemic amyloidosis. Instead, a correlation between serum lipid and hemoglobin levels existed in this specific group. Moreover, the current study demonstrated that serum LDL-C levels in patients with nephrotic syndrome are associated with the etiology of disease (lower levels in patients with systemic AA amyloidosis compared to primary glomerulonephritis).
Systemic AA amyloidosis is the most frequent type of systemic amyloidosis around the world, predominantly in developing countries.Citation3,Citation4 Renal biopsy registries in our country also revealed that systemic AA amyloidosis was one of the most frequent cause of amyloidosis (12.2–16.8%).Citation16,Citation17 The leading cause of systemic AA amyloidosis in our study was the FMF (51.3%) which is compatible with other studies reported from Turkey (30.5–73%).Citation8,Citation18
Dyslipidemia is a typical feature of nephrotic syndrome at presentation, irrespective of glomerular disease etiology. Its prevalence is about 90% in both primary glomerulonephritis and systemic AA amyloidosis. However, the pattern of the dyslipidemia differs from each other in these two distinct etiologies. Serum levels of LDL-C and HDL-C are lower in systemic AA amyloidosis; they are independently associated with serum hemoglobin levels; but none of them is associated with proteinuria or serum albumin levels which is a prominent feature of PG-related lipid abnormalities. About one-third of systemic AA amyloidosis patients had normal LDL-C, whereas about half of them had low HDL-C.
In many clinical studies, it is proposed that increased synthesis and decreased catabolism of the serum lipids, due to hypoalbuminemia and proteinuria, cause dyslipidemia in case of nephrotic syndrome.Citation10,Citation13–15 This theory probably explains the lipid changes seen in a primary disease that is limited to the kidney. However, this may not be the unique mechanism responsible for dyslipidemia seen in systemic AA amyloidosis that has different pathophysiological mechanisms, namely deposition of fibrillary protein. First of all, systemic AA amyloidosis is generally a systemic disease unlike most of the PG which are usually limited to the kidney.Citation7–9 Secondly, systemic AA amyloidosis develops from sustained inflammatory diseases taking years unlike PG which usually begin with abrupt onset.Citation7 Thirdly, endothelial dysfunction may be more prominent in systemic AA amyloidosis compared to PG.Citation19 Finally, in systemic AA amyloidosis, underlying disease, the duration of the disease and intestinal involvement can take part in changes of disease-related parameters. All these pathophysiological differences between systemic AA amyloidosis and PG bring into mind the question that whether they are also responsible for the different phenotypes of dyslipidemia recognized in both the diseases.
We found that all serum lipid parameters except HDL-C were related with serum total protein level, albumin level and proteinuria level in patients with PG. This was not valid for patients with systemic AA amyloidosis. Dyslipidemia in nephrotic syndrome characterized by elevations of TC, LDL-C, TG and lipoprotein(a).Citation10,Citation13–15 Despite these findings, HDL-C have been reported to be elevated, normal or decreased.Citation20–23 It is known that, this marked increase in plasma cholesterol level and LDL-C is usually due to increased synthesis and decreased catabolism.Citation9,Citation10,Citation13–15 Other principal contributor of elevated levels of cholesterol in nephrotic syndrome is increased hepatic lipogenesis.Citation14,Citation15 The stimulants which liver responds are probably hypoalbuminemia and decreased plasma oncotic pressure.Citation13,Citation15 On the other hand, proteinuria itself may also be a stimulant for liver to increase the lipogenesis. And lipolytic enzyme losses with proteinuria can contribute to decrease catabolism of lipids.Citation14,Citation23 Elevation of hepatic HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase and LDL-receptor deficiency are potential responsible pathways for increased levels of TC and LDL-C.Citation9,Citation10 Urinary losses of LCAT (lecithin-cholesterol acyltransferase), reduced hepatic HDL-receptors, down regulation of lipoprotein lipase and hepatic triglyceride lipase are responsible for abnormalities in the metabolisms of HDL-C and triglyceride-rich lipoproteins.Citation9,Citation10 In the present study, serum lipid parameters were correlated with serum total protein, albumin and proteinuria in patients with PG, compatible with previous studies.
Possible explanation that why there was no such relation in patients with systemic AA amyloidosis may be from the systemic nature of the disease. Also inflammation and catabolic process of the disease may affect lipid metabolism in patients with systemic AA amyloidosis. Although we did not find correlation between serum lipids and CRP levels, several studies reported that inflammation can alter serum lipids.Citation24,Citation25 Patients with systemic AA amyloidosis also had lower levels of hemoglobin, MCV, MPV and higher platelet count. All these parameters can be reflection of more severe inflammation in systemic AA amyloidosis. Unfortunately, we cannot investigate SAA, interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and other inflammatory cytokines that may potentially and indirectly influence serum lipid levels. Interestingly, we found that LDL-C, HDL-C and TG levels were independently associated with hemoglobin levels in overall patients. To the best of our knowledge, there is no study investigated such association. A potential mechanism in this correlation may be related to the decreased plasma oncotic pressure due to hypoalbuminemia that lead to hemoconcentration, and this may correlate with the degree of the lipidemia.
Few studies evaluated the dyslipidemia basically in systemic AA amyloidosis, unlike PG. It was proposed that hypercholesterolemia in amyloidosis is less common than in other forms of the nephrotic syndrome.Citation9 Paydas reported that mean level of serum cholesterol of the 59 patients with systemic AA amyloidosis was between 7.77 and 10.36 mmol/L.Citation26 In this report, frequency and nature of dyslipidemia were not defined, and it was noted that proteinuria was found to be correlated with lipidemia. Study population of this report also included both normal and reduced renal function. Yilmaz et al. investigated the relationship between ADMA (asymmetric dimethyl-arginine) levels, proteinuria and endothelial dysfunction in proteinuric patients with systemic AA amyloidosis and PG.Citation19 There was no significant difference between patients with systemic AA amyloidosis and PG with regard to serum TC, LDL-C, HDL-C and TG levels, and frequency of dyslipidemia was not reported. However, it is not clear whether the measurement of serum lipid levels was made at diagnosis or while the patients were on maintenance therapy. Lipid parameters (TC, LDL-C and TG) did not correlate with proteinuria in both the groups. Moreover, correlation of serum lipid parameters with serum albumin was not assessed in that study. Cengiz et al. reported that the serum levels of TC, LDL-C, HDL-C and TG in patients with amyloid-chronic kidney disease were significantly higher than in patients with chronic kidney disease without amyloidosis.Citation27 They did not find any correlation between serum lipid parameters and serum albumin, urine protein levels or GFR. Population of this study included patients with reduced renal function which may alter the levels of serum lipid parameters. In our study, we found that dyslipidemia, mainly elevated serum LDL-C, was present in majority of the patients with secondary amyloidosis. There was no clear correlation between lipid parameters and serum albumin or urinary protein levels. When considering these previous studies evaluating clinical and biochemical characteristics of patients with systemic AA amyloidosis, the present study had some important and pioneer features. We analyzed the lipid parameters of nephrotic amyloid patients with normal GFRs at admission, and the comparison was made with nephrotic syndrome patients due to PG.
Several limitations of our study should also be discussed. Cross-sectional design of the study limited us to evaluate the levels of serum AA and some interleukins, malnutrition degree of patients, or hepatic and endocrine system involvement of amyloidosis. Hence, it was not possible to explain the associations of serum lipid parameters and serum albumin, proteinuria and CRP levels in patients with systemic AA amyloidosis in detail. Secondly, we did not investigate properties of protein and lipid excretion in urine of the patients, which may be dissimilar for these two different pathophysiological disease groups.
In conclusion, majority of the patients with systemic AA amyloidosis and PG have serum lipid abnormalities. Although dyslipidemia is closely associated with levels of serum albumin and proteinuria in PG, it is not the case for systemic AA amyloidosis. Mechanisms of dyslipidemia in secondary amyloidosis may be more complex than the known classical mechanisms. Further studies are desirable to convey exact pathophysiology of lipid abnormalities seen in systemic AA amyloidosis, and those studies may enlighten the decision and modalities of the treatment of dyslipidemia.
References
- Virchow VR. Ueber einem Gehirn and Roeckenmark des Menschen auf Gefundene Substanz mit chemischen reaction der Cellulose. Virchows Arch Pathol Anat. 1854;6:135–138
- Glenner GG. Amyloid deposits and amyloidosis. The beta-fibrilloses. N Engl J Med. 1980;302(23):1283–1292
- Dilsen N, Konice M, Aral O, Ocal L. The prevalence, importance and significance of amyloidosis with rheumatic diseases in Turkey. In: Natwig JB, Forre O, Husby G, et al. eds. Amyloidosis. Dordrecht: Kluwer; 1990:870–873
- Simms RW, Prout MN, Cohen AS. The epidemiology of AL and AA amyloidosis. Baillieres Clin Rheumatol. 1994;8(3):627–634
- Falk RH, Skinner M. The systemic amyloidoses: An overview. Adv Intern Med. 2000;45:107–137
- Cunnane G. Amyloid proteins in pathogenesis of AA amyloidosis. Lancet. 2001;358(9275):4–5
- Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–2371
- Tuglular S, Yalcinkaya F, Paydas S, et al. A retrospective analysis for aetiology and clinical findings of 287 secondary amyloidosis cases in Turkey. Nephrol Dial Transplant. 2002;17(11):2003–2005
- Appel GB, Radhakrishnan J, D’Agati V. Secondary glomerular disease. In: Brenner BM, ed. The Kidney. Philadelphia, PA: Saunders; 2008:1100–1104
- Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63(5):1964–1976
- Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J Am Soc Nephrol. 2006;17(4 Suppl 2):S145–S147
- Mallick NP, Short CD. The nephrotic syndrome and ischemic heart disease. Nephron. 1981;27:54–57
- Appel GB, Blum CB, Chien S, Kunis CL, Appel AS. The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N Engl J Med. 1985;312(24):1544–1548
- Warwick GL, Caslake MJ, Boulton-Jones JM, Dagen M, Packard CJ, Shepherd J. Low-density lipoprotein metabolism in the nephrotic syndrome. Metabolism. 1990;39(2):187–192
- Joven J, Villabona C, Vilella E, Masana L, Albertí R, Vallés M. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N Engl J Med. 1990;323(9):579–584
- Hur E, Taskin H, Bozkurt D. Adult native renal biopsy experience of Ege University for 12 consecutive years. BANTAO J. 2010;8(1):22–29
- Piskinpasa S, Akoglu H, Koc E, et al. Revisiting secondary amyloidosis for an inadequately investigated feature: Dyslipidemia. Rheumatol Int. 2013;33(4):993–999
- Ensari C, Ensari A, Tumer N, Ertug E. Clinicopathological and epidemiological analysis of amyloidosis in Turkish patients. Nephrol Dial Transplant. 2005;20(8):1721–1725
- Yilmaz MI, Sonmez A, Saglam M, et al. ADMA levels correlate with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol. 2008;19(2):388–395
- Chan MK, Persaud JW, Ramdial L, Varghese Z, Sweny P, Moorhead JF. Hyperlipidaemia in untreated nephrotic syndrome, increased production or decreased removal? Clin Chim Acta. 1981;117(3):317–323
- Ohta T, Matsuda I. Lipid and apolipoprotein levels in patients with nephrotic syndrome. Clin Chim Acta. 1981;117(2):133–143
- Michaeli J, Bar-On H, Shafrir E. Lipoprotein profiles in a heterogeneous group of patients with nephrotic syndrome. Isr J Med Sci. 1981;17(11):1001–1008
- Vaziri ND, Sato T, Liang K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int. 2003;63(5):1756–1763
- Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: A population based cross sectional study. BMJ. 1996;312(7038):1061–1065
- Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young, apparently healthy men: Associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism. 2003;52(9):1113–1116
- Paydas S. Report on 59 patients with renal amyloidosis. Int Urol Nephrol. 1999;31(5):619–631
- Cengiz K, Bakan A, Yilmaz H. Lipoprotein abnormalities in patients with secondary renal amyloidosis. Int Urol Nephrol. 2001;32(4):615–619
