Abstract
Background: The risk of contrast-induced acute kidney injury (CIAKI) is significantly increased in patients with diabetes mellitus. This study aimed to investigate molecular mechanisms of contrast media-induced apoptosis in diabetic rat kidneys, especially the involvement of ERK1/2 and JNK signal pathways. Methods: Diabetic Sprague–Dawley rats were induced by intraperitoneal injection of streptozotocin. Ten weeks later the normal and diabetic rats were administered high-osmolar contrast media (HOCM; meglumine diatrizoate) or normal saline (10 mL/kg) injection for 2 consecutive days. At 24 h after the operation, the rats were sacrificed, the blood samples were collected for examining serum creatinine and the kidneys were collected for determining the expression of caspase-3 by immunohistochemistry and the expression of Bcl-2, Bax, upstream signal molecule p-JNK, and p-ERK1/2 by western blotting. Results: The serum creatinine was significantly increased in diabetes + contrast media group (DC group) after operation compared with in the diabetic group (D group; 103.89 ± 9.01 μmol/L vs. 71.52 ± 7.03 μmol/L, p < 0.05). While creatinine clearance rate (Ccr) was significantly decreased in DC group after operation (1.49 ± 0.33 mL/min vs. 2.60 ± 0.54 mL/min, p < 0.05). Especially, in the diabetic kidney, the expression of caspase-3 was also significantly increased after intravenous injection of HOCM compared with normal saline. The expression level of upstream signal molecule p-JNK protein was apparently increased, but p-ERK1/2 protein was significantly decreased (both p < 0.05). Conclusions: The ionic HOCM-induced renal cells apoptosis in diabetic rats through activating the caspase-3 apoptotic pathway, which might be mediated by upstream MAPK (inhibiting p-ERK1/2 expression and promoting p-JNK expression) signal pathways.
Introduction
Despite adherence to risk assessment and prevention strategies, contrast-induced acute kidney injury (CIAKI) remains the third leading cause of hospital-acquired acute renal failure, especially for those patients undergoing percutaneous coronary intervention.Citation1 And, it is closely related to prolonged hospitalization and higher rates of in-hospital, 1- and 5-year mortality.Citation2–4 Patients with diabetes are at particularly high risk of developing CIAKI. A recent meta-analysis found 43 possible risk factors for CIAKI, but diabetes was the only independent predictor of CIAKI.Citation5 In addition, compared with non-diabetic patients, diabetic patients with CIAKI had a significantly decreased survival rate over a 2-year follow-up.Citation6
At present, the mechanisms of CIAKI remain unclear, reactive oxygen species generation and direct cytotoxicity effects are considered to be the most likely mechanisms.Citation7–10 Several in vitro studies showed that high-glucose condition enhanced the susceptibility of renal cell to the cytotoxic effects of both the high- and low-osmolar contrast media (HOCM and LOCM). Meanwhile, both contrast media and hyperglycemia (30 mmol/L glucose) were associated with a significantly increased level of apoptosis. Thus, the combination of these effects had a synergistic effect on accentuating renal tubular apoptosis and therefore, it increased the risk of CIAKI.Citation11,Citation12
Different extracellular stimuli induces activation of multiple members of the mitogen activated protein kinases (MAPK) including c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), extracellular signal-regulated kinase1/2 (ERK1/2), p38 kinase, and the phosphoinositide 3-kinase (PI3K)/Akt pathway.Citation13–15 Lee et al.Citation16,Citation17 demonstrated that contrast media can activate the JNK/ATF2 pathway which plays a pivotal role in contrast media-induced cytotoxicity (apoptosis) in both in vitro and in vivo experiments. Although most studies showed that ERK could deliver survival signals that protect cells from apoptosis,Citation15 but there are also some opposite evidence.Citation18–20 Therefore, the contribution of ERK to renal cell apoptosis is controversial. Especially, its role in contrast media-induced apoptosis remains unclear.
Accordingly, in this study, we compared the differences in the induction of apoptosis between diabetic and normal rats after ionic HOCM administration. Further, we examined the expression of ERK1/2 and JNK in the diabetic and normal rat kidneys after administrating ionic HOCM to determine their roles in contrast media-induced apoptosis.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats with the age of 8–10 weeks old (weighing 250 ± 20 g) were purchased from Animal Experimental Center of Academy of Military Medical Sciences. Rats were housed at 23–25 °C in a 12-h light/dark cycle with free access to standard rat chow and tap water. All animal experiments were performed with the permission from the Medical Ethics Committee of Tianjin Medical University and followed the protocol outlined in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Animal model and study design
After 1 week of acclimatization, diabetes was induced in SD rats by a single streptozotocin (STZ) injection (Sigma Company, St. Louis, MO) at a dose of 60 mg/kg (i.p., dissolved in citrate buffer) after overnight fast. After 72 h, rats with fasting blood glucose more than 16.7 mmol/L were considered successfully induced for diabetes. The normal group rats were treated with citrate buffer. The blood glucose of diabetic rats was monitored once a week. All rats were fed with a regular diet and water till 10 weeks after STZ injection. The diabetic rats were randomly divided into two groups including STZ-induced diabetic group (D group) and diabetes + HOCM group (DC group); the non-STZ-induced rats as control group were also randomly divided into two groups including normal control group (N group) and HOCM group (NC group; each group, n = 6). The ionic HOCM, meglumine diatrizoate (DTZ 60%, Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China) at a dose of 10 mL/kg was administered into the femoral vein once daily for 2 consecutive days (day 1 and day 2) to groups DC and NC, and the same volume of normal saline was administered to N group or D group under 10% chloral hydrate (300 mg/kg) anesthesia.
Baseline blood samples were collected from the femoral vein before the first administration of DTZ or normal saline under 10% chloral hydrate anesthesia (day 1). All rats were kept in individual metabolism cages and deprived of water for 24 h but allowed free access to chow after the last administration (day 2). The final blood sample was collected from inferior vena cava and both kidneys were excised at the end of the study (day 3). The right kidneys were preserved for renal histopathological examination. The left kidneys were placed in separate Eppendorf tubes after washing in ice-cold normal saline and stored at −80 °C till the day of analysis.
Biochemical studies and renal function assessment
The chemicals and kits were supplied from BioSino Bio-technology and Science Inc. (Beijing, China). Serum and urinary creatinine measurements were performed with the Jaffe method. Ccr was calculated by U × V/P [where U = urine creatinine (mg/dL), V = urine volume (mL/min/100 g), and P = serum creatinine (mg/dL)], and was expressed as mL/min/100 g body weight.
Caspase-3 immunohistochemical staining
Immunohistochemistry (Streptavidin–biotin–peroxidase complex staining) for caspase-3 protein was performed that involved blocking endogenous peroxidase with 0.3% hydrogen peroxide, followed by pre-incubation in blocking serum and then incubation with the primary antibody: caspase-3 (rabbit, Boster, Wuhan, Hubei, China) overnight at 4 °C. After washing in phosphate-buffered saline (PBS), the biotinylated secondary antibody was applied for 30 min at room temperature followed by streptavidin horseradish–peroxidase complex. Then the sections were rinsed with PBS again and visualized by incubation with 0.05% (w/v) 3,3′-diamino-benzidine tetrahydrochloride dehydrate. Slides were counterstained with 0.5% methyl green before cover slipping. The value of optical density is monitored by computer-assisted image analysis. For each slide, 10 fields were randomly chosen. The value of mean optical density was calculated. The assays were performed in a blinded manner.
Western blotting analysis
Protein of renal tissue was extracted by total protein extraction buffer on ice for 30 min. An equal amount of protein was loaded onto a 10% SDS denaturing polyacrylamide gel, separated by electrophoresis, transferred onto PVDF membrane (Merck Millipore, Billerica, MA). Non-specific binding sites were blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20. The membranes were incubated with the specific primary antibody overnight at 4 °C. Then the membranes were washed three times and subsequently incubated with the secondary antibody conjugated to horseradish peroxidase (HRP). Protein was visualized using enhanced chemiluminescence. Protein levels of Bcl-2, Bax, p-ERK1/2, and p-JNK were expressed as ratio to levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The anti-GAPDH antibody was purchased from Abcam Inc. (Cambridge, UK). The anti-Bcl-2, Bax, p-ERK1/2, and p-JNK antibodies were purchased from Cell Signaling Technology Inc. (Boston, MA). The resulting bands were quantified using Gene Tools software (Syngene, Frederick, MD).
Statistical analysis
Data were expressed as means ± SD values. Continuous variables among groups were analyzed by one-way ANOVA followed by the least-significant difference procedure. All analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL). p < 0.05 was considered statistically significant.
Results
Renal function parameters
Diabetic rats showed similar levels of serum creatinine, but little higher rates of creatinine clearance as compared to the normal group. The ionic HOCM induced increased serum creatinine levels and decreased Ccr in the normal rats, but the difference did not reach statistical significance. Compared with D group, DC group had significantly higher levels of serum creatinine (103.89 ± 9.01 μmol/L vs. 71.52 ± 7.03 μmol/L, p < 0.05), but significantly lower Ccr (1.49 ± 0.33 mL/min vs. 2.60 ± 0.54 mL/min, p < 0.05; ).
Table 1. Renal function parameters in the study groups (x ± s).
Effect on the expression of apoptosis-related protein caspase-3
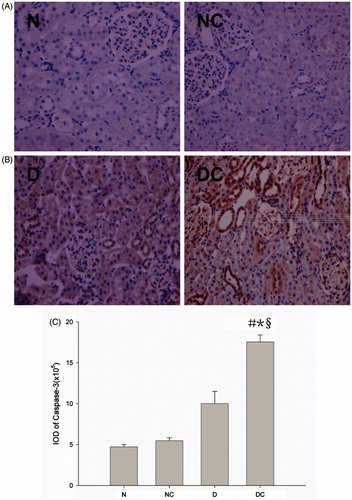
As shown in , the caspase-3 protein expression in kidney tissue at 24 h after intravenous injection was determined. Diabetic rats showed numerically higher levels of caspase-3 expression than normal rats after intravenous injection of normal saline, but the difference did not reach statistical significance. The ionic HOCM induced increased caspase-3 expression in normal kidney, but the difference did not reach statistical significance. Especially, in the diabetic kidney, the expression of caspase-3 was significantly increased after intravenous injection of HOCM compared with normal saline (p < 0.05).
Figure 1. Effect of contrast media on apoptosis-related protein caspase-3 activation. Representative immunohistochemical findings (magnification 400×) of caspase-3 in normal rat kidney (A) and diabetic rat kidney (B) after normal saline (NS) or meglumine diatrizoate (DTZ) treatment. The meglumine diatrizoate induced increased caspase-3 expression in normal kidney, but the difference did not reach statistical significance (A,C). Especially in the diabetic kidney, the expression of caspase-3 was also significantly increased after intravenous injection of DTZ compared with normal saline (p < 0.05) (B,C). IOD = integrated optical density #p < 0.05 versus N group; *p < 0.05 versus NC group; §p < 0.05 versus D group.
Effects on the expression of Bcl-2 and Bax
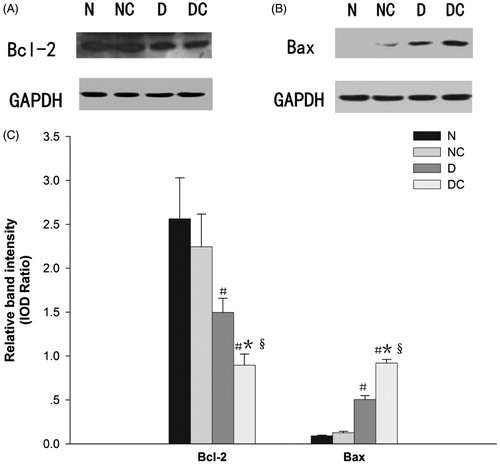
shows anti-apoptosis protein Bcl-2 and pro-apoptosis protein Bax expression in homogenized kidney tissue. The ionic HOCM induced increased Bax and decreased Bcl-2 expression in normal kidney, although the difference did not reach statistical significance. HOCM resulted in a significant reduction in Bcl-2 protein expression and opposite increase in Bax expression in the diabetic kidney (both p < 0.05). Furthermore, diabetic rats had higher Bax and lower Bcl-2 expression levels than normal rats in the contrast media-treated group (both p < 0.01).
Figure 2. Effect of contrast media on Bcl-2 family proteins expression. Representative western blot findings of Bcl-2 (A) and Bax (B) in the kidney of each group. Diabetic rats had higher Bax and lower Bcl-2 expression than normal rats in the contrast media treated group (both p < 0.01) (C). The expression of Bcl-2 was also significantly decreased and the expression of Bax was significantly increased in the diabetic kidney after intravenous injection of meglumine diatrizoate compared with normal saline (both p < 0.05) (C). IOD = integrated optical density #p < 0.05 versus N group; *p < 0.05 versus NC group; §p < 0.05 versus D group.
Effects on the expression of p-ERK1/2 and p-JNK
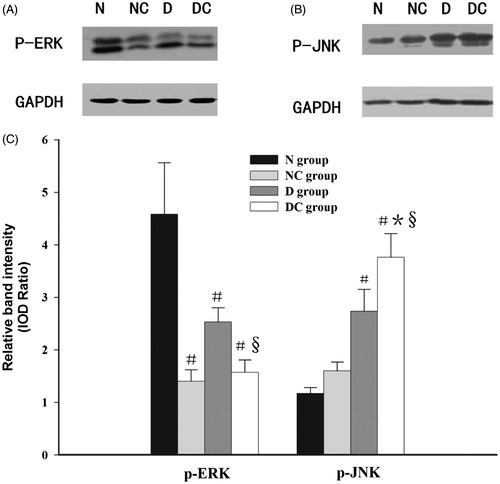
As shown in , the HOCM induced increased p-JNK expression in normal kidney, although the difference did not reach statistical significance. Especially, the expression of p-JNK was also significantly increased in the diabetic kidneys after intravenous HOCM injection compared with normal saline (p < 0.05), however, the expression of upstream signal molecule p-ERK1/2 was just the opposite (p < 0.05). Moreover, the expression of upstream signal molecule p-JNK in diabetic kidney was significantly higher than that of normal rats in the contrast media-treated group (p < 0.05).
Figure 3. Effect of contrast media on phosphorylation of ERK1/2 and JNK. Representative western blot findings of p-ERK1/2 (A) and p-JNK (B) in the kidney of each group. The expression of p-JNK was significantly increased in the diabetic kidney after intravenous injection meglumine diatrizoate compared with normal saline (p < 0.05) (B,C), however, the expression of upstream signal molecule p-ERK1/2 was just the opposite (p < 0.05) (A,C). Moreover, the expression of upstream signal molecule p-JNK in diabetic kidney was significantly higher than that of normal rats in the contrast media-treated group (p < 0.05) (B,C). IOD = integrated optical density #p < 0.05 versus N group; *p < 0.05 versus NC group; §p < 0.05 versus D group.
Discussion
In this study, we established a reliable experimental model of CIAKI in diabetic rats to observe the differences in the induction of apoptosis and the phosphorylation of ERK1/2 and JNK in the diabetic and normal rat kidney. Our results showed that intravenous continuous administration of ionic HOCM could induce acute kidney injury. We also observed that there was a higher extent of apoptosis in diabetic than in normal rats. Furthermore, contrast media could induce apoptosis through activating the caspase-3 mediated apoptotic pathway. Finally, contrast media could influence MAPK signal pathways, including the inhibition of p-ERK1/2 expression and promotion of p-JNK expression in diabetic kidney.
Clinical studies have confirmed that diabetes mellitus is one of the most important risk factors for CIAKI.Citation6,Citation21 Recent evidence suggests that patients with chronic kidney disease even in a pre-diabetic state, defined by a fasting glucose between 100 to 125 mg/dL, are at increased risk for CIAKI. Therefore, the hyperglycemia itself makes the kidney more susceptible to CIAKI.Citation22 Several in vitro studies alsoCitation11,Citation12 demonstrated that high-glucose condition enhanced the susceptibility of renal cells to the cytotoxic effects (apoptosis) of both the HOCM and LOCM. In other words, the combination of high-glucose and contrast media has a synergistic effect on accentuating renal tubular apoptosis and therefore increasing the risk of CIAKI. Lee et al.Citation17 also demonstrated that ionic LOCM induced more apoptosis in diabetic than did in normal rat kidneys. In accordance with previous studies, our research also showed that intravenous continuous administration of ionic HOCM-induced acute kidney injury, and there was a higher extent of apoptosis in diabetic than in normal rats. Compared with in NC group, the serum creatinine was significantly increased in DC group after operation (p < 0.05), while the Ccr was also decreased in the DC group after operation, although the difference did not reach statistical significance. Moreover, the expression of apoptosis-related protein caspase-3 was significantly increased in the diabetic kidneys after intravenous injection of HOCM compared with in normal kidneys.
Previous in vitro studiesCitation23,Citation24 showed that apoptosis of renal tubular epithelial cells can be induced by iodinated contrast medium and caspase-3 is the primary executioner caspase during the execution phase of apoptosis. In a rat model of CIAKI, cellular injury of the renal medulla consisted of extensive DNA fragmentation, which has been attributed to medullary hypoxia.Citation25,Citation26 Our study also confirms that contrast media induces renal cell apoptosis through activating the caspase-3-mediated apoptotic pathway especially in diabetic rats. It is well known that the mitochondrial pathway of apoptosis is regulated by Bcl-2 family members. Hence, we also studied the expression of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 after incubation with contrast media. Several in vitro studiesCitation16,Citation26–29 demonstrated that contrast media could induce renal cell apoptosis through mediating Bcl-2 family expression. Yano et al.Citation26 have shown that contrast media-induced apoptosis in the porcine tubular cell line, LLC-PK-1, and that the injuries might be due to de-regulation in Bax/Bcl-2 expression, followed by an increase in caspases-9 and -3 activities in vitro. In accordance with the previous observations, we found that contrast media could upset the balance between Bax and Bcl-2 expression, inducing an increase of pro-apoptotic protein Bax and a decrease of anti-apoptotic protein Bcl-2.
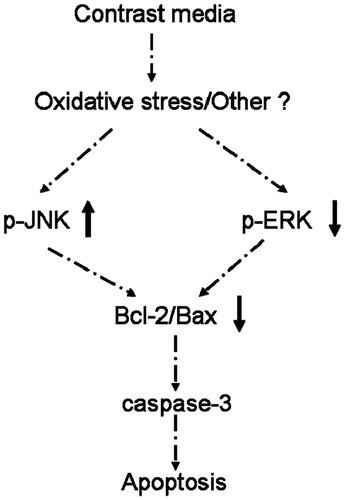
As mentioned before, the protective effect of ERK in apoptosis is controversial,Citation15,Citation18–20 while its role in contrast media-induced apoptosis remains unclear. In this in vivo study, although intravenous injection of HOCM induced increased expression of p-JNK in normal rats, but the difference did not reach statistical significance. Notably, the expression of p-ERK1/2 was significantly decreased after intravenous injection of HOCM in normal rats, which suggests that the drop of p-ERK1/2 was more prominent than the rise of p-JNK. In other words, for normal rats, reduced expression of p-ERK1/2 by contrast media might play a more important role than the rise of p-JNK in contrast media-induced apoptosis. In addition, compared with normal rats, diabetic rats showed higher p-JNK expression, but lower p-ERK1/2 expression. Meanwhile, intravenous injection of HOCM further induced higher expression of p-JNK and lower expression of p-ERK1/2 significantly in diabetic kidney. Moreover, the patterns of induced p-ERK1/2 and p-JNK expression in diabetic and normal rats were similar to those of the induced apoptosis-related proteins caspase-3. Therefore, our study also provided the evidence that the ERK pathway could deliver survival signals that protect cells from apoptosis, in contrast, the JNK pathway was associated with induction of apoptosis. Given all that, ERK1/2 and JNK expression might be involved in iodinated contrast media-induced renal cell apoptosis in diabetic rats. Based on the findings of our study and others,Citation11,Citation12,Citation15–17,Citation26–29 we propose a pathophysiologic mechanism for apoptosis induced by ionic HOCM in diabetic rats (). According to these findings, ERK1/2 and JNK pathway may be a target for novel therapeutic intervention in CIAKI.
Figure 4. Pathophysiologic mechanisms for the apoptosis induced by ionic high-osmolar contrast media in diabetic rats.
Several study limitations should be addressed. First, time-dependent changes of the expression of p-ERK1/2 and p-JNK were not measured in this study. Second, the effects of low-osmolar and iso-osmolar contrast media on apoptosis in kidney tubular cells were not assessed. However, this should not have any significant impact on our main findings.
In summary, this work provides new evidence that ionic HOCM can activate the ERK1/2 and JNK signaling pathways that further induce renal cell apoptosis through activating the caspase-3-mediated apoptotic pathway. ERK1/2 signaling pathway may play a protective role in contrast media-induced apoptosis in both normal and diabetic kidneys. Accordingly, this study provides new insight into the mechanism and prevention of CIAKI.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–195
- Finn WF. The clinical and renal consequences of contrast-induce nephropathy. Nephrol Dial Transplant. 2006;21:i2–i10
- Mautone A, Brown JR. Contrast-induced nephropathy in patients undergoing elective and urgent procedures. J Interv Cardiol. 2010;23:78–85
- Katzberg RW, Haller C. Contrast-induced nephrotoxicity: Clinical landscape. Kidney Int Suppl. 2006;100:S3–S7
- Reed PS, Boura JA, O'Neill WW, Kahn JK. Comparison of the usefulness of gadodiamide and iodine mixture versus iodinated contrast alone for prevention of contrast-induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. Am J Cardiol. 2007;100:1090–1093
- Zaytseva NV, Shamkhalova MS, Shestakova MV, et al. Contrast-induced nephropathy in patients with type 2 diabetes during coronary angiography: Risk-factors and prognostic value. Diabetes Res Clin Pract. 2009;86(Suppl 1):S63–S69
- Fanning NF, Manning BJ, Buckley J, Redmond HP. Iodinated contrast media induce neutrophil apoptosis through a mitochondrial and caspase mediated pathway. Br J Radiol. 2002;75:861–873
- Hardiek K, Katholi RE, Ramkumar V, Deitrick C. Proximal tubule cell response to radiographic contrast media. Am J Physiol Renal Physiol. 2001;280:F61–F70
- Zager RA, Johnson AC, Hanson SY. Radiographic contrast media-induced tubular injury: Evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;64:128–139
- Briguori C, Tavano D, Colombo A. Contrast agent-associated nephrotoxicity. Prog Cardiovasc Dis. 2003;45:493–503
- Wasaki M, Sugimoto J, Shirota K. Glucose alters the susceptibility of mesangial cells to contrast media. Invest Radiol. 2001;36:355–362
- O'Donnell DH, Moloney MA, Bouchier-Hayes DJ, Lee MJ. Contrast-induced nephrotoxicity: Possible synergistic effect of stress hyperglycemia. AJR Am J Roentgenol. 2010;195:W45–W49
- Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296
- Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414
- Yang JY, Michod D, Walicki J, Widmann C. Surviving the kiss of death. Biochem Pharmacol. 2004;68:1027–1031
- Lee HC, Sheu SH, Yen HW, Lai WT, Chang JG. JNK/ATF2 Pathway is involved in iodinated contrast media-induced apoptosis. Am J Nephrol. 2010;31:125–133
- Lee HC, Chang JG, Yen HW, Liu IH, Lai WT, Sheu SH. Ionic contrast media induced more apoptosis in diabetic kidney than nonionic contrast media. J Nephrol. 2011;24:376–380
- Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2004;287:F543–F549
- Kim YK, Kim HJ, Kwon CH, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol. 2005;25:374–382
- Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem. 2002;277:43377–43388
- Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444–449
- Toprak O, Cirit M, Yesil M, et al. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:819–826
- Hizoh I, Haller C. Radiocontrast-induced renal tubular cell apoptosis: Hypertonic versus oxidative stress. Invest Radiol. 2002;37:428–434
- Heinrich MC, Kuhlmann MK, Kohlbacher S, et al. Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: In vitro study. Radiology. 2007;242:425–434
- Beeri R, Symon Z, Brezis M, et al. Rapid DNA fragmentation from hypoxia along the thick ascending limb of rat kidneys. Kidney Int. 1995;47:1806–1810
- Yano T, Itoh Y, Sendo T, Kubota T, Oishi R. Cyclic AMP reverses radiocontrast media-induced apoptosis in LLC-PK1 cells by activating A kinase/PI3 kinase. Kidney Int. 2003;64:2052–2063
- Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29:2569–2576
- Yano T, Itoh Y, Kubota T, et al. A prostacyclin analog prevents radiocontrast nephropathy via phosphorylation of cyclic AMP response element binding protein. Am J Pathol. 2005;166:1333–1342
- Kolyada AY, Liangos O, Madias NE, Jaber BL. Protective Effect of erythropoietin against radiocontrast-induced renal tubular epithelial cell injury. Am J Nephrol. 2008;28:203–209
