Abstract
This study aims to investigate the role of urinary biomarkers in the determination of the potential risks of renal parenchymal tubular damage in adult patients who underwent percutaneous nephrolithotomy (PNL) with the indication of renal stone. A randomized and prospective controlled study was performed between June and December 2013. We enrolled 29 consecutive patients with renal calculi > 2 cm and who underwent PNL, as well as 47 healthy control subjects. Urine samples, including 2 h before surgery, 2 and 24 h after surgery were collected from the patient group. Freshly voided urine samples were collected from the control group. Kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-glucosaminidase (NAG), and liver-type fatty acid binding protein (LFABP) levels were measured from these urine samples. The mean KIM-1/Cr value that measured 24 h after the operation was statistically significant, higher than its preoperative (preop) level (p = 0.045). A significant difference was detected between the mean preop and postoperative (postop) 24 h NAG/Cr values (p < 0.001). Also, postop 24 h NGAL/Cr levels were statistically significant, higher than its preop levels (p = 0.013). According to the comparison of preop and postop levels, an increase in LFABP/Cr values secondary to surgical intervention was observed without any statistically significant difference. Besides the LFABP/Cr levels do not change after percutaneous kidney surgery, KIM-1/Cr, NAG/Cr, and NGAL/Cr levels increase postop period, especially at 24 h. Further studies with a larger series and repeated measurements should be performed to clarify if they can be used to demonstrate renal damage after percutaneous surgery or not.
Introduction
Nephrolithiasis is a serious problem worldwide. In recent studies, an increase in the incidence of the stone disease has been observed. According to EAU guidelines, in the treatment of renal stones larger than 2 cm, percutaneous nephrolithotomy (PNL) is the gold standard. Moreover, in the management of cystine stones, stones refractory to shock wave lithotripsy (SWL), and residual stones after open surgery/SWL, PNL is recommended.Citation1 Although PNL is accepted as a minimally invasive, safe procedure, postoperative (postop) acute kidney injury (AKI) has been observed in a few patients.Citation2
PNL may cause vascular injury, hemodynamic deterioration, and parenchymal damage. Currently, there is no adequate imaging modality for assessing parenchymal injury; thus, a novel diagnostic test that can reliably detect such injuries is needed. Recent studies have revealed that certain patients undergoing unilateral PNL suffer from AKI after surgery. If not detected in time, AKI can lead to a series of clinical problems.Citation3,4 In recent years, many molecules have been used in various laboratory and animal studies to detect renal injury in its earlier stages. Serum creatinine measurements have been used most commonly for evaluating renal functions. However, serum creatinine does not demonstrate the parenchymal renal injury. Given that serum creatinine begins to rise 48–72 h after renal injury and only after the loss of nearly 50% of renal function, it is inadequate as a marker for detecting renal damage.Citation5,6 Therefore, a clinically usable, non-invasive, and reliable biomarker that will detect parenchymal damage and AKI during an early post-PNL period is needed. The current literature data suggest that the most effective biomarkers of AKI are kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-glucosaminidase (NAG), and liver-type fatty acid binding protein (LFABP), which is upregulated in proximal tubule cells after ischemic or nephrotoxic AKI.Citation7,8
KIM-1, a type 1 cell membrane glycoprotein is expressed in humans, is a sensitive and specific biomarker of proximal tubule injury. It was detected in renal tubular epithelial cells when screening for molecules involved in the pathogenesis of AKI.Citation7 Human NGAL was originally identified as a 25-kDa protein bound covalently to gelatinase from neutrophils.Citation9 NAG is a lysosome hydrolase with a molecular weight of approximately 140,000 Da, and it cannot be filtered normally through the glomeruli.Citation2,10 L-FABP, an intracellular carrier protein of free fatty acids, is expressed in the proximal tubules of the human kidney.Citation11 Following tubular damage, different molecules are released from damaged proximal tubular cells. These molecules can be used for monitoring tubular epithelial cell damage.Citation12
Though PNL procedures are used widely around the world, the effect of PNL on renal parenchyma has not been elucidated yet. In addition, a urinary biomarker that can specifically demonstrate renal parenchymal damage is not available yet. Although urinary markers such as KIM-1, NAG, NGAL, and L-FABP have been used in many studies, their use in patients who have undergone PNL procedures has not been investigated in any study. Early detection of the level of renal damage and prediction of the AKI potential are very important prognostic factors because they will facilitate initiation of treatment as soon as possible.
The objective of this study is to investigate the role of urinary biomarkers for determining the potential risks of renal parenchymal injury due to PNL in adult patients with the indication of renal stones. Our study is the first large-scale prospective study on this issue.
Material and methods
This study was approved by the Institutional Research Ethics Board (Medical Faculty, Dicle University). A randomized and prospective controlled study was performed between June and December 2013, and informed consent was obtained from all subjects before their participation in the study. We enrolled 29 consecutive patients with renal calculi > 2 cm and who underwent PNL, as well as 47 control patients who had no urogenital disease based on the result of tests and ultrasonographic examinations. Patients with serious hydronephrosis, a serious disease; renal insufficiency; obesity; emaciation; renal ectopia; horseshoe kidney; functional or organic solitary kidney; or patients with incomplete clinical data or incomplete samples were excluded from this study.
Patients’ demographic data related to age, sex, and stone size were collected. The results of preoperative (preop) tests such as routine blood tests, serum biochemistry, urinalysis, urine culture, ultrasonography, and plain X-ray were recorded. Preoperatively, antibiotics were administered according to the results of urine cultures. Computerized tomography was performed routinely to analyze the size and location of stones, anatomical structure, and to determine the targeted calyx. All patients included in the study provided a spontaneously voided urine sample 2 h before PNL treatment (preop). Additionally, these patients provided urine samples at 2 (postop 2 h) and 24 h (postop 24 h) following PNL. Volunteers provided medical history information and one freshly voided urine sample. Urine creatinine levels were measured using autoanalyzer Architect C 16000 (Abbott Laboratories, Abbott Park, IL, USA).
Urine NGAL, KIM-1, NAG, and L-FABP levels were determined using a commercially available quantitative sandwich immunoassay technique (SunRed Biotechnology Company, Shanghai, China). Urine samples were stored at 4 °C and centrifuged for 5 min at 3000 rpm within 1 week of their collection. The supernatant was used for measurement. For standardization, the recorded measurements of KIM-1, NAG, NGAL, and L-FABP levels were divided by urine creatinine levels, and depended measurements were compared.
All patients who underwent PNL were performed with 30-F sheath and same tract. PNL was performed using a 26-F rigid nephroscope. The stones were fragmented with a pneumatic lithotripter. Fragments accessible via the rigid nephroscope were cleared with grasping forceps. Intactness of the collecting system was evaluated intraoperatively via fluoroscopic imaging and antegrade nephrostography. The procedure was completed by placing an 18-F Foley catheter into the calyx.
Statistical analysis
The mean (± standard deviation), median, maximum, and minimum values of the data were calculated. Compatibility of variables with the normal distribution pattern was investigated using the Kolmogorov–Smirnov and Shapiro–Wilk tests. The data that were not compliant with the normality of the distribution were compared using the Mann–Whitney U test for determining the difference between the two groups. Categorical values were compared using the χ2 test. Correlations between two numerical variables were investigated using Spearman correlation analysis. For statistical analyses, the SPSS (SPSS Inc., Chicago, IL) software package was used. p < 0.05 was accepted as the cut-off value for the level of statistical significance.
Results
The characteristics of the study participants are summarized in . The patient population consisted of 17 men and 12 women (total n: 29), while the control group (n: 47) comprised 25 male and 12 female subjects. Mean ages of the patient and control groups were 37.3 ± 19.5 and 40.5 ± 21.4 years, respectively. The patients suffered from left (n:15) and right (n: 14) kidney stones. The mean stone burden was 95.8 ± 174.7 mm2. The patients underwent standard PNL procedures.
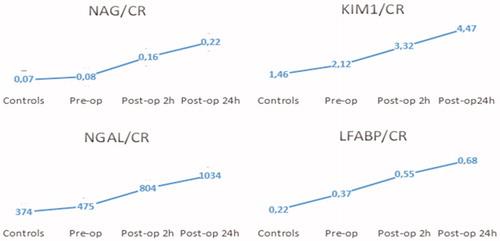
Mean (± SD) concentrations of KIM-1, NGAL, NAG, and LFABP are listed in , and statistical comparisons of the split ratio to creatinine levels of KIM-1, NGAL, NAG, and LFABP are given in .
Table 1. Comparison between PNL and control groups.
Table 2. Early renal injury markers in patient with urolithiasis who underwent percutaneous nephrolithotripsy (PNL).
Table 3. Differences (p values) in the early kidney injury markers/creatinine ratios between the first measurements and the control groups.
The mean KIM-1/Cr value (3.32 ± 1.78) measured immediately after PNL was higher than its preop (2.12 ± 0.98) value without any statistically significant difference between the two values (p > 0.05). However, the mean KIM-1/Cr value (4.47 ± 2.41) measured 24 h after the operation was statistically significant, higher than its preop value (p = 0.001).
The mean NAG/Cr value (0.16 ± 0.11) at the postop 2 h was higher than that in the preop (0.08 ± 0.028) measurement without any significant difference. Similarly, any significant difference was not found between the mean preop NAG/Cr value of patient group and control group (0.07 ± 0.02). However, a significant difference was detected between the mean preop and postop 24 h NAG/Cr values (p = 0.001).
The mean urinary NGAL/Cr ratio (804 ± 215) of the patients at postop 2 h value was considerably higher than the preop measurement (475 ± 173) without any statistically significant difference. A significant difference was observed between the postop 24 h (1034 ± 662) and the preop values (p = 0.013).
An increase in LFABP/Cr values secondary to surgical intervention was observed without any statistically significant difference among the repeated measurements. Urinary kidney injury markers/creatinine ratios measured in the control and patient group with preop and postop values are shown in .
Discussion
With its lower mortality and higher success rates, PNL has been recently preferred as a minimally invasive technique for treating renal stones over open surgery. Although PNL is a minimally invasive method, postop complications such as vascular or parenchymal bleeding, hemodynamic impairment, and AKI can be observed. Experimental animal studies have demonstrated that PNL did not affect renal morphology and functions, except for the possibility of development of a minimal scar along the nephrostomy tract.Citation13 Moskovitz et al. performed post-PNL dynamic renal scanning with DMSA and detected a mild decrease in total functional volume and a statistically significant drop in regional renal function.Citation14 Conventional imaging modalities do not always detect renal damage that occurs after PNL operation. As a laboratory parameter of renal injury, serum creatinine levels rise long after the operation, and they are influenced by many factors, including the patient age and the presence of acute renal failure (ARF) and chronic renal failure. Therefore, non-invasive, reliable, and easily applicable diagnostic tests that can detect the severity of renal damage after renal injury are needed.
In recent years, many studies were performed on laboratory parameters to detect renal damage in the early postop period. In these studies, mostly, potential non-invasive biomarkers such as urinary proteins KIM-1, NGAL, L’FABP, NAG, IL-18, clusterin, and osteopontin were investigated.Citation7,8,15 Basic characteristics related to urinary biomarkers are summarized in . Among these potential biomarkers, KIM-1 is the most frequently confirmed and promising marker. When compared with other biomarkers that predict proximal tubular toxicity, urinary KIM-1 has the highest sensitivity and specificity levels.Citation16 KIM-1, a type-1 transmembrane protein, was originally discovered as a putative epithelial cell-adhesive molecule containing a novel immunoglobulin domain, which was absent in normal condition but was elevated in the proximal tubule apical membrane cells after injury.Citation17–19 Urinary KIM-1 levels are strongly related to tubular KIM-1 expression in experimental and human renal disease.Citation18 In a study which was performed on rats, Ichimura et al. confirmed that urinary KIM-1 is a more valuable and selective biomarker than serum creatinine level for predicting kidney injury.Citation19 However, the sensitivity and specificity of urinary KIM-I for predicting the outcome of renal injury have been demonstrated in human studies as well.Citation20 In two recently published systematic reviews, it has been reported that KIM-1 was an effective and reliable urinary biomarker for diagnosing AKI within 24 h of renal injury.Citation21,22 Clinical studies have demonstrated the excessive tubular production of KIM-1 in FSGS, IgA nephropathy, and MPGN. Moreover, a linear and proportional correlation was revealed between increased KIM-1 levels, proteinuria, development of tubular damage, and interstitial fibrosis secondary to proteinuria.Citation23–25 Han et al. suggested that significantly increased urinary KIM-1 levels are found in patients with ischemic ATN and asserted that KIM-1 levels increase dramatically within 12 h after ischemic injury. As a concluding remark, they indicated that KIM-1 could be used as a biomarker for predicting ATN risk.Citation26 In their study involving patients with obstructive uropathy, Warelewska et al. reported significant increases in KIM/Cr levels secondary to obstruction and surgical intervention.Citation27 Furthermore, owing to its predictive value as a biomarker with higher sensitivity and specificity, the US FDA and European Drug Agency have approved use of KIM-1 for monitoring drug-induced nephropathy.Citation28
Table 4. Basic characteristics of some urinary biomarkers.
In our study, KIM-1/Cr levels at 2 h and 24 h post-PNL were higher than those found in the control group, which consisted of healthy individuals; however, only the 24 h KIM-1/Cr ratios were found to be statistically significant. In addition, a significant difference was detected between the preop and postop 24 h KIM-1/Cr levels, and the 24 h KIM-1/Cr levels were considerably higher than those estimated 24 h after the operation. This finding implies that KIM-1, which was accepted as a predictive biomarker for renal damage in previous studies, can, in fact, be an important biomarker for predicting early stage renal injury. In our study, similar to the study performed by Warelewska et al., relatively higher urinary KIM-1/Cr ratios were estimated in patients with obstructive uropathy secondary to the presence of a renal stone when compared with the control group comprising healthy individuals. Ichimura et al. could not detect KIM-1 in the urine samples of healthy children, but Warelewska et al. revealed the presence of scarce amounts of KIM-1 in the urine samples of healthy children.Citation12,27 Similar to the outcomes of the study performed by Wasilewska et al., we detected lower urinary concentrations of KIM-1 in the control group. Based on the data obtained in recent studies, urinary clearance of KIM-1 in many chronic proteinuric conditions has been associated with renal fibrosis and inflammation.Citation25 In this case, KIM-1 cannot be regarded as a non-invasive biomarker only for proximal tubuli, but it should be considered as a potential biomarker for predicting chronic tubulointerstitial injury. In our study, a progressive increase in KIM-1/Cr levels was observed following PNL. Therefore, to predict the degree of renal injury secondary to PNL, KIM-1 levels can be measured during the post-PNL period. In screening scientific papers related to this issue, we could not find any study demonstrating the degree of post-PNL kidney injury. Our study is the first investigation conducted on this issue. Because we measured KIM/Cr levels at 24 h postop at the latest, the postop duration of increased KIM/Cr levels and the time of regression to normal levels can be determined only with periodic measurements.
Human NGAL was originally identified as a 25-kDa protein bound covalently to gelatinase from neutrophils. NGAL is normally expressed at very low levels in several human tissues, including the kidneys, lungs, stomach, and colon. NGAL expression is induced markedly in injured epithelia.Citation29,30 Urinary NGAL is an especially promising predictive biomarker of ARF. Indeed, it is upregulated within 2 h after cellular damage in ARF.Citation31 In microassay analyses, NGAL has shown the earliest and the most prominent increase following ischemic and toxic renal injury.Citation32 Mishra et al. performed a study in patients with cardiopulmonary bypass and reported that postop urinary NGAL levels predicted the risk of ARF development long before increases in serum creatinine levels.Citation5 In their study on patients with obstructive uropathy, Wasilewska et al. demonstrated a correlation between NGAL levels and the degree of obstruction and found significant increases in NGAL levels after surgical intervention.Citation27 Similarly, in our study, we observed significant increases in urinary NGAL level when compared with the preop value, which we attributed to renal parenchymal damage. This finding indicates that NGAL can be used to predict renal parenchymal injury during the early phase of AKI.
L-FABP is a protein expressed in the proximal tubule of the kidney. Increased expression and urinary excretion have been described in animal models of AKI.Citation33 In children who developed AKI after undergoing cardiopulmonary bypass, urine L-FABP concentrations increased significantly within 4 h of the surgery.Citation34 In our study, increases in the post-PNL L-FABP/Cr values were detected at a non-statistically significant level.
NAG has attracted increasing attention as a biomarker. Widely distributed in various tissues and cells in the body, NAG is a lysosome hydrolase with a molecular weight of approximately 140,000 Da, and it cannot be filtered normally through the glomeruli.Citation10 It is distributed mainly in lysosomes in the epithelial cells of nephric tubules, and small amounts of it are found in the mitochondria. For patients with the non-glomerular disease without marked albuminuria, urinary NAG originates mainly from the nephric tubules. Because dynamic concentrations of urinary NAG change with urine flow and the collection of 24 h urine samples is a complicated procedure, the NAG/creatinine ratio has been used to avoid errors due to random measurement at a single time point. NAG is not inactivated easily in the urine, and urinary NAG output is relatively stable under normal conditions. Moreover, urinary NAG increases significantly during necrosis of the tubular epithelium, and this change occurs much earlier than do changes in blood urea nitrogen and Cr.Citation35 Jiang et al. demonstrated that urine NAG levels were more sensitive than serum creatinine levels for predicting AKI development in the early postop period of unilateral PNL operations.Citation2 Moreover, in the current study, we observed progressive increases in urine NAG levels during the post-PNL period. Furthermore, a statistically significant difference was detected between postop 24 h NAG and preop NAG levels Therefore, we believe that during the early post-PNL period, NAG levels can be used to indicate both the severity of renal injury and the level of renal function. When patients with renal stones and the control group were compared, higher levels of the four biomarkers were detected in patients with renal stones. We opine that levels of urinary biomarkers increase in patients with renal stones and or hydronephrosis.
This is the first study to report on this issue. Consequently, this study has some limitations. First of all, a small number of patients were included. Furthermore, repeated measurement of these AKI markers are lacking. Forty-seven control patients, unlike the study group, had not received anesthesia. Since this is an initial study that explored the acute effects of PNL on urinary AKI markers, and limited kits were used in this study, future studies on this issue should incorporate large number of patients with more repeated measurements. Determining a good biomarker for kidney injury may give us a new vision on renal stone surgery such as making a decision on the duration or calibration of PNL procedure or define the exact reoperation time in patients with residual stones.
Conclusion
Besides the LFABP/Cr levels did not change after percutaneous renal stone surgery, KIM-1/Cr, NAG/Cr and NGAL/Cr levels increased postop period, especially at 24 h. Larger series with repeated and late measurements should be performed to clarify this issue further. By this way, some urinary biomarkers can be used to clinically demonstrate renal parenchymal damage and AKI which may lead us to perform different surgical techniques and make a decision on operation time or repeated treatment times.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Türk KT, Petrik A, Sarica K, et al. Guidelines on urolithiasis. European Association of Urology; 2010
- Jiang C, Qi C, Sun K, Xia L, Xue W, Huang Y. Diagnostic value of N-acetyl-β-D-glucosaminidase for the early prediction of acute kidney injury after percutaneous nephrolithotripsy. Exp Ther Med. 2013;5(1):197–200
- Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10(3):R73
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury following cardiac surgery. Lancet 2005;365(9466):1231–1238
- Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23(2):194–200
- Ichimura T, Mou S. Kidney injury molecule-1 in acute kidney injury and renal repair: A review. Zhong Xi Yi Jie He Xue Bao. 2008;6(5):533–538
- Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–273
- Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15(12):3073–3082
- Ye RG, Xu HS. The diagnosis value of urinary lysozyme and N-acetyl beta glucosaminidase in renal tubulointerstitial diseases. Chin J Pract Intern Med. 1999;19:198–199
- Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47(3):439–444
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F563
- Traxer O, Smith TG III, Pearle MS, Corwin TS, Saboorian H, Cadeddu JA. Renal parenchymal injury after standard and mini percutaneous nephrostolithotomy. J Urol. 2001;165(5):1693–1695
- Moskovitz B, Halachmi S, Sopov V, et al. Effect of percutaneous nephrolithotripsy on renal function: Assessment with quantitative SPECT of (99m)Tc-DMSA renal scintigraphy. J Endourol. 2006;20(2):102–106
- Ting YT, Coates PT, Walker RJ, McLellan AD. Urinary tubular biomarkers as potential early predictors of renal allograft rejection. Nephrology (Carlton). 2012;17(1):11–16
- Zhou Y, Vaidya VS, Brown RP, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101(1):159–170
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244
- Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220(1):7–16
- Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142
- Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl. 2008;241:78–83
- Huang Y, Don-Wauchope AC. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: A systematic review. Inflamm Allergy Drug Targets. 2011;10(4):260–271
- Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int. 2008;73(9):1008–1016
- Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: Human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134
- Bonventre JV. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268
- van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217
- Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882
- Wasilewska A, Taranta-Janusz K, Debek W, Zoch-Zwierz W, Kuroczycka-Saniutycz E. Kim-1 and NGAL: New markers of obstructive nephropaty. Pediatr Nephrol. 2011;26:579–586
- Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: A dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28(5):455–462
- Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: Pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207(4):663–672
- Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482(1-2):298–307
- Thomas AA, Demirjian S, Lane BR, et al. Acute kidney injury: Novel biomarkers and potential utility for patient care in urology. Urology. 2011;77(1):5–11
- Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: Clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80(4):365–376
- Negishi K, Noiri E, Doi K, et al. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174(4):1154–1159
- Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472
- D’Amico G, Bazzi C. Urinary protein and enzyme excretion as markers of tubular damage. Curr Opin Nephrol Hypertens. 2003;12(6):639–643