Abstract
Atherosclerosis-induced premature vascular diseases are the leading cause of mortality among patients with chronic kidney disease (CKD). The pathogenetic mechanism of atherosclerosis in patients with CKD has not been fully explained. Experimental studies have demonstrated that high dietary sodium intake not only increases circulatory volume and blood pressure, but also facilitates development of atherosclerosis by reducing production-bioavailability of nitric oxide due to oxidative stress and accordingly by enhancing endothelial and arterial stiffness. In this study, we investigated the relationship between sodium consumption and carotid artery intima-media thickness, which is the indicator of atherosclerosis, by determining daily urinary sodium excretion, which is a reliable indicator of sodium consumption, in our patient group. Our patient group included 193 patients with stage 2–4 non-diabetic CKD and without a history of atherosclerotic disease. We determined that 77% of our patients have been consuming more than 2 g of sodium per day, which is the upper limit of sodium consumption recommended for patients with CKD. We determined a positive linear correlation between carotid artery intima-media thickness and patient age (p < 0.001), C-reactive protein (p < 0.001), urinary sodium excretion (p < 0.001), body mass index (p = 0.002), systolic blood pressure (p = 0.002), hemoglobin (p = 0.030), triglycerides (p = 0.043), and diastolic blood pressure (p = 0.049). We also found a negative linear correlation between carotid artery intima-media thickness and glomerular filtration rate (p = 0.008). We found that urinary sodium excretion is the determinant of intima-media thickness even if all factors associated with intima-media thickness are adjusted, and that intima-media thickness increases by 0.031 (0.004–0.059) mm per 2 g increase in daily sodium excretion, independent from overall factors (p = 0.025). Our results reveal a relation between urinary sodium excretion and carotid artery intima-media thickness and suggest that excessive sodium consumption predisposes development of atherosclerosis in patients with CKD.
Introduction
Chronic kidney disease (CKD) is a common worldwide public health problem with high morbidity and mortality.Citation1,Citation2 Atherosclerosis-induced premature vascular diseases are the leading cause of mortality among patients with CKD.Citation2 An increase in mortality appears in the early stages of the disease in patients with CKD and the mortality rate gradually increases in association with the degree of renal dysfunction. It has been reported that the 5-year mortality rate in stage 2 CKD cases is 20%, and even after stratification by gender, age, and diabetes, and that cardiovascular mortality in patients with end-stage renal disease is 10–20 times higher as compared to the general population.Citation3,Citation4 Traditional atherosclerotic risk factors, such as advanced age, increased blood pressure, diabetes mellitus (DM), lipid disorders, obesity, and tobacco consumption have failed to fully explain early-rapid development of atherosclerosis and the related high morbidity and mortality rates in CKD patients.Citation5 Determining the factors that influence the development of atherosclerosis in CKD cases could substantially reduce the risk of cardio-cerebrovascular disease and mortality rates in CKD patients.Citation5,Citation6
Experimental studies have demonstrated that excessive dietary sodium intake not only increases circulatory volume and blood pressure,Citation7,Citation8 but also facilitates development of atherosclerosis by reducing production-bioavailability of nitric oxide (NO) due to oxidative stress and by enhancing endothelial and arterial stiffness.Citation9,Citation10–14 Despite conflicting data,Citation15 it has been reported that excessive sodium consumption enhances the development of atherosclerotic events, such as ischemic heart disease and stroke.Citation16–19
To our knowledge, there have been no previous studies investigating the relationship between daily sodium consumption and carotid artery intima-media thickness (IMT), which is the non-invasive marker of atherosclerosis and measured via ultrasonographic method, in CKD patients. The amount of dietary sodium intake and daily urinary sodium excretion has been shown to be equal to each other in a steady state. A determination of the amount of 24-h urinary sodium excretion, therefore, is the best method for determining daily dietary sodium intake.Citation20,Citation21 We investigated the relationship between sodium consumption and atherosclerosis by determining daily urinary sodium excretion in our patient group. This group consisted of patients with stage 2–4 non-DM chronic kidney disease and no history of atherosclerotic disease or salt-losing nephropathy.
Materials and methods
The study protocol was approved by the Trakya University Local Ethics Committee. Ambulatory patients between 18 and 65 years old with a glomerular filtration rate (GFR) of 15–90 mL/min 1.73 m2 (patients with stage 2–4 CKD) and a body mass index (BMI) of 18.5–35.0 kg/m2, who were being followed in our polyclinic and developed CKD independent of non-diabetes mellitus, had no salt-losing nephropathy or history of malignancy or cardio-cerebrovascular disease or any acute disease, were invited to participate in the study. All patients were informed about the study verbally and in writing and the patients who gave written consent were included in the study.
Daily urine volume, height without shoes, and weight of the patients were recorded at the beginning of the study. The patients’ BMI values were calculated using the formula weight (kg)/height (m)2. Body surface area was calculated using the formula 0.007184 × height (cm)0.725 × weight (kg)0.425. Blood pressure was measured using a calibrated aneroid sphygmomanometer that had a cuff width and balloon diameter suitable for each patient, in accordance with the World Health Organization’s (WHO) measurement criteria.Citation22 A detailed anamnesis was taken for each patient and smoking status, status of renin-angiotensin-aldosterone system (RAS) inhibitors, and statins, and acetylsalicylic acid use were recorded.
The study group consisted of a total of 193 CKD patients: 109 females and 84 males. Twelve (6.2%) of the patients were active smokers. Ninety (46.6%) of the patients had been receiving renin-angiotensin-aldosterone system (RAS) inhibitors, 33 (17.1%) had been receiving statin, and 28 (14.5%) had been receiving acetylsalicylic acid. The causes of CKD in this group varied as follows: 60 patients (31.1%) developed CKD as the result of chronic glomerulonephritis, 58 (30%) had chronic tubulointerstitial nephritis that progressed to CKD, 50 patients (25.9%) developed CKD as a complication of hypertension, 22 (11.4%) had autosomal dominant polycystic kidney disease that progressed to CKD, and 3 patients (1.5%) developed CKD as the result of amyloidosis.
We divided our patients according to the recommendations of the Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Guideline.Citation23 One group was comprised of those with a daily dietary sodium intake of a maximum of 2 g (Normal Sodium Group, urinary sodium excretion <85 mEq/day), and the other group was comprised of those consuming more than 2 g of sodium per day (Excessive Sodium Group, urinary sodium excretion ≥85 mEq/day). The Normal Sodium Group consisted of 44 CKD patients (29 females and 15 males). In this group, three patients (6.8%) were active smokers. Twenty-one patients (47.7%) had been receiving RAS inhibitors, 11 patients (25%) had been receiving statin, and six patients (13.6%) had been receiving acetylsalicylic acid. In this group, CKD had developed due to chronic glomerulonephritis in 11 patients (25.0%), chronic tubulointerstitial nephritis in 17 patients (30.1%), hypertension in 13 patients (29.5%), autosomal dominant polycystic kidney disease in 2 patients (4.5%), and amyloidosis in 1 patient (2.3%). The Excessive Sodium Group consisted of 149 CKD patients (80 females and 69 males). Nine of these patients (6%) were active smokers. Sixty-nine patients (46.3%) had been receiving RAS inhibitors, 22 patients (14.8%) has been receiving statin, and 22 patients (14.8%) had been receiving acetylsalicylic acid. In this group, CKD had developed due to chronic glomerulonephritis in 49 patients (32.9%), chronic tubulointerstitial nephritis in 41 patients (27.5%), hypertension in 37 patients (24.8%), autosomal dominant polycystic kidney disease in 20 patients (13.4%), and amyloidosis in 2 patients (1.3%).
We also divided the patients into groups according to the presence of subclinical atherosclerosis.Citation24 The group without subclinical atherosclerosis had carotid artery IMT < 750 mm, and the group with subclinical atherosclerosis had carotid artery IMT ≥ 750 mm. The group without subclinical atherosclerosis consisted of 88 CKD patients (55 females and 33 males). Three patients (3.4%) in this group were active smokers. Forty-six patients (52.3%) had been receiving RAS inhibitors, 12 patients (13.6%) had been receiving statin, and 11 patients (12.5%) had been receiving acetylsalicylic acid. In this group, CKD had developed due to chronic glomerulonephritis in 31 patients (35.2%), chronic tubulointerstitial nephritis in 26 patients (29.5%), hypertension in 22 patients (25%), autosomal dominant polycystic kidney disease in 7 patients (8.0%), and amyloidosis in 2 patients (2.3%). The group with subclinical atherosclerosis consisted of 105 CKD patients (54 females and 51 males). Nine of the patients in this group (8.6%) were active smokers. Forty-four patients (41.9%) had been receiving RAS inhibitors, 21 patients (20.0%) had been receiving statin, and 17 patients (16.2%) had been receiving acetylsalicylic acid. In this group, 29 patients (27.6%) developed CKD due to chronic glomerulonephritis, 32 patients (30.5%) developed it due to chronic tubulointerstitial nephritis, 28 patients (26.7%) due to hypertension, 15 patients (14.3%) due to autosomal dominant polycystic kidney disease, and 1 patient (1%) developed CKD due to amyloidosis.
Biochemical analysis
Serum fasting blood glucose (FBG), urea, creatinine, uric acid, albumin, total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides (TG), sodium, potassium, calcium, and phosphate concentrations were measured using the Siemens Advia 1800 device (Munich, Germany). Urinary albumin excretion (UAE), urinary protein excretion (UPE), and urinary sodium excretion (UNaE) in 24-h urine samples were determined using the spectrophotometric method. GFR was calculated using the following formula: 175 × serum creatinine−1.154 × age−0.203 × serum urea−0.170 × serum albumin−0.318 × 0.762 (for women). A complete blood count was performed using the Siemens Advia 2120i device (Munich, Germany). Patients’ intact parathyroid hormone (iPTH) concentration was determined using the chemical immunoassay method performed on the Siemens Immulite 2000 device (Munich, Germany).
Blood samples drawn from the antecubital vein between 8:00 and 10:00 in the morning after 10 h of fasting were put into plain biochemistry tubes. They were immediately centrifuged at 5000 rpm for 5 min. Serums were derived and put into polypropylene tubes and stored at −80 °C. These samples were used to measure high sensitive C-reactive protein (hs-CRP) by the micro ELISA method in the Bio-Tek Instruments (Winooski, VT) Microplate EL 309 auto reader device using the EIA test kit (Lot no: RN-45504, DRG Diagnostics, Marburg, Germany).
Measurement of carotid artery intima-media thickness
Carotid artery IMT measurements were performed in the Trakya University Faculty of Medicine, Department of Radiology using the B mode of the EsoatMyLab60 Xvision device (Genoa, Italy). Patients were in the supine position and their heads were placed in the extension position while two different measurements were performed on the left main carotid artery and the right main carotid artery (1 cm proximal of the bulb). The mean value of these two measurements was calculated. The measurement was not performed in the places where atheroma plaque was observed. The space between the two echogenic lines observed between the intima–lumen interface and the media-adventitia interface was considered to be the carotid artery IMT. The mean carotid artery intima-media thickness was calculated by dividing the total value of the right and left carotid artery intima-media thicknesses by two.
Statistical analyses
The study data were transferred to a computer and a statistical analysis was performed using STATISTICA AXA 7.1 (Tulsa, OK) (License No: AXA507C775506FAN3) in the Trakya University Faculty of Medicine Deanship, Data Processing Center. First, the Kolmogorov–Smirnov test was performed in all groups and for all data collected. The suitability of data for normal distribution was assessed. The difference between the parametric data of the two independent groups was analyzed using Student’s t-test in case the data were distributed normally, and using the Mann–Whitney U test in case the data were not distributed normally. The difference between the categorical data of the two independent groups was analyzed using a chi-square test. The relationship between carotid artery IMT and other parametric data was analyzed using the Pearson Correlation test in case both groups of data were distributed normally and using the Spearman Correlation test in case at least one of the groups of data was not distributed normally or was categorical. A linear regression test (method: stepwise) was used to analyze causality between IMT and other data. In statistical analyses, values of p < 0.05 were considered significant.
Results
Comparison of data of the CKD patients with daily sodium excretion <85 mEq (Normal Sodium Group) and the patients with daily sodium excretion ≥85 mEq (Excessive Sodium Group)
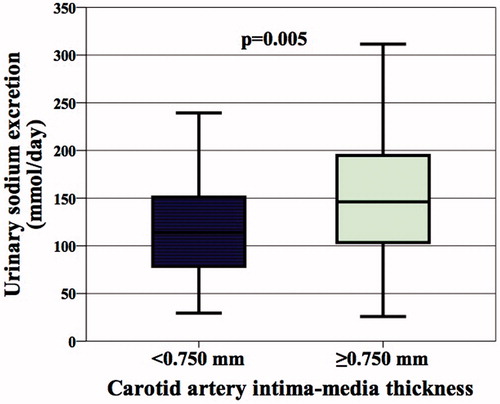
The demographic, clinical, and laboratory data for the patients who received sodium more or less than 85 mEq/day (approximately 2 g/day), which is the upper limit recommended by the WHO for healthy adults and by the KDIGO guideline for CKD patients, are demonstrated in . We determined that daily sodium intake was over 2 g in 77% of the patients. Mean sodium excretion in 24-h urine samples was 63 mmol (1.5 g) in the patients whose sodium consumption was within the normal ranges and 158 mmol (3.6 g) in the patients who were consuming excessive sodium (p < 0.001, ). The distribution of the etiological factors that lead to CKD, the rates of statin, acetylsalicylic acid, and RAS inhibitors use, mean age, gender distribution, and rate of smoking were statistically similar in both groups. The mean carotid artery IMT was 0.066 mm (9%) higher in the group consuming excessive sodium as compared to the group consuming sodium within the normal ranges (p = 0.029). In addition, SBP (p = 0.007), DBP (p = 0.006), MAP (p = 0.006), and creatinine (p = 0.009) values were higher in the group with excessive sodium consumption as compared to the group with normal sodium consumption. Both groups have similar serum FBG, uric acid, urea, lipid, sodium, potassium, calcium, phosphate, and iPTH values as well as urinary protein and albumin excretion, leucocytes, platelets, hemoglobin, and mean GFR values.
Figure 1. Carotid artery intima-media thickness (in mm) of the normal sodium group (urinary sodium excretion <85 mmol/day) and the excessive sodium group (urinary sodium excretion ≥85 mmol/day). Note: Data are mean with 95% confidence interval.
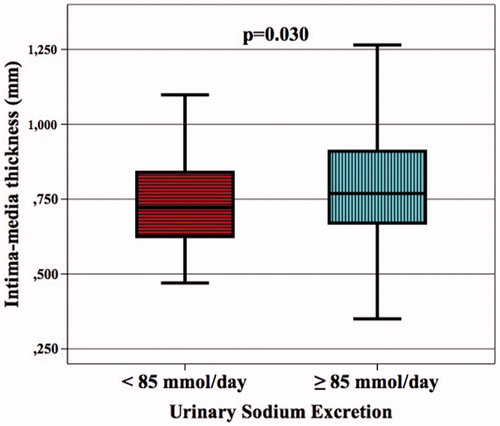
Table 1. Demographic, clinical, and laboratory parameters of the patients.
Comparison of data for the CKD patients with carotid artery IMT < 750 mm and ≥750 mm
Demographic, clinical, and laboratory data for the patients with an IMT value < 750 mm (the group without subclinical atherosclerosis) and the patients with an IMT value ≥ 750 mm (the group with subclinical atherosclerosis) is given in . Fifty-four percent of the patients developed subclinical atherosclerosis. The mean IMT was 0.627 mm in the patients without subclinical atherosclerosis and 0.902 mm in the patients with subclinical atherosclerosis (p < 0.001, ). The distribution of the etiological factors that lead to CKD, the rates of statin, acetylsalicylic acid, and RAS inhibitors use, gender distribution, and rate of smoking were statistically similar in both groups. The mean sodium excretion in 24-h urine samples was 26 mEq (0.6 g) higher in the patients with subclinical atherosclerosis as compared to the patients without subclinical atherosclerosis (p = 0.005). In addition, patient age (p < 0.001), BMI (p = 0.009), SBP (p = 0.008), MAP (p = 0.019), urea (p = 0.042), and serum hs-CRP (p < 0.001) were also higher in the patients with subclinical atherosclerosis as compared to the patients without subclinical atherosclerosis. The mean GFR value was lower in the patients with subclinical atherosclerosis as compared to the patients without subclinical atherosclerosis (p = 0.016). Both groups had similar serum FBG, uric acid, creatinine, lipid, sodium, potassium, calcium, phosphate, and PTH values as well as urinary protein and albumin excretion and leucocytes, platelets, and hemoglobin values.
Evaluation of multiple correlations between carotid artery intima-media thickness and other data
There was a positive linear correlation between carotid artery IMT and patient age, hs-CRP, amount of sodium excretion in 24-h urine samples, BMI, SBP, MAP, hemoglobin, triglycerides, and DBP. Moreover, there was a negative linear correlation between mean IMT and GFR. The data that have a statistically significant correlation with the mean carotid artery IMT are shown in . In addition to those correlations, a positive linear correlation was determined between IMT and smoking (r = 0.137, p = 0.057) and a negative linear correlation was determined between IMT and using RAS inhibitors (r = −0.127, p = 0.079), even though neither of these correlations were statistically significant. The relationship between carotid artery IMT and UNaE is illustrated in .
Figure 3. Relationship between carotid artery intima-media thickness and urinary sodium excretion. Scattergram, showing the relation between carotid artery intima-media thickness (in mm) and urinary sodium excretion (in mmol/day) in patient with chronic kidney disease.
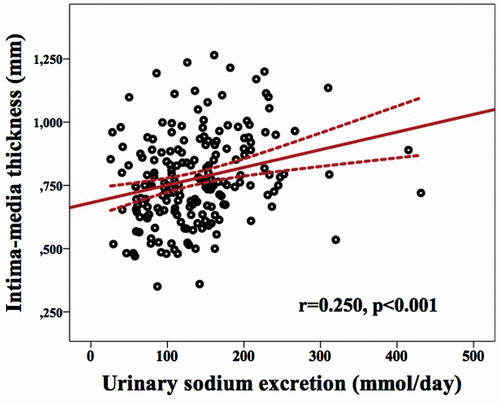
Table 2. Univariate associations of covariates with carotid intima-media thickness.
Evaluation of the causal relationship between daily urinary sodium excretion and carotid artery intima-media thickness
We performed a linear regression analysis to investigate the independent effect of the amount of daily urinary sodium excretion on the increase in carotid artery IMT. Patient age, hs-CRP, 24-h urinary sodium excretion, BMI, SBP, GFR, hemoglobin, and triglycerides, which we determined to be associated with carotid artery IMT (a dependent variable), were determined to be independent variables. In the model, we created (adjusted R2 = 0.339, p < 0.000001), we found that the amount of daily urinary sodium excretion is an independent determinant of IMT and that IMT is increased by 0.031 (0.004–0.059) mm per 2 g (85 mmol) increase in daily urinary sodium excretion even after all the above-mentioned factors have been adjusted (p = 0.025). Again in this model, we observed that patient age, hs-CRP, hemoglobin, GFR, and SBP values are independent determinants of carotid artery IMT in addition to urinary sodium excretion. The results of the Multivariate Linear Regression analyses for carotid artery intima-media thickness are demonstrated in .
Table 3. Multivariate linear regression analyses for carotid artery intima-media thickness in patients.
Discussion
In this study, we found a positive linear correlation between urinary sodium excretion and carotid artery IMT in the patients whose CKD was not the result of DM and did not have known atherosclerotic disease or salt-losing nephropathy. Moreover, we determined that urinary sodium excretion is the independent determinant of IMT, even when adjusting for patient age, hs-CRP, BMI, SBP, GFR, hemoglobin, and triglycerides, which we determined to be associated with IMT. To our knowledge, this is the first study to determine that urinary sodium excretion, which is a reliable indicator of daily dietary sodium intake, associated to an increase in IMT, which is a non-invasive indicator of atherosclerosis, in CKD patients.
Chronic kidney disease unfavorably influences the public health because of increasing prevalence all over the world as well as unacceptable high mortality rate.Citation2,Citation3 It was determined that the most important causes of increased morbi-mortality in patients with chronic kidney disease are atherosclerosis-related cardio-cerebrovascular diseases.Citation2,Citation4–6 Determining carotid artery IMT via ultrasonography is considered a cheap, easily applicable, and repeatable non-invasive method for assessing the presence and extensiveness of atherosclerosis.Citation25,Citation26 O’Leary et al.Citation26 determined that the risk of developing myocardial infarction and stroke increases by 47% per 0.200 mm increase in IMT in 4476 patients aged 65 years and older. It has been demonstrated that carotid artery IMT is also an independent marker of cardiovascular mortality in patients receiving dialysis for the treatment of end-stage kidney disease.Citation24 Coronary artery disease was detected in 7% of the patients with carotid artery IMT < 0.750 mm and 73% of the patients with carotid artery IMT ≥ 0.750 mm among CKD patients who underwent a coronary angiography for the purpose of preparation for renal transplantation.Citation24 The results of this study revealed that the specificity and sensitivity of carotid artery IMT > 0.750 mm are 90% and 73%, respectively, in determining coronary artery disease. In this respect, IMT ≥ 0.750 mm was considered a diagnostic marker of subclinical atherosclerosis.Citation24 In the present study, the presence of subclinical atherosclerosis in more than half of the patients with a GFR value of 15–84 mL/min 1.73 m2 suggests that the risk of morbidity-mortality is substantially higher in patients who had no history of cardio-cerebrovascular disease or no cardiac complaint.Citation24,Citation26
Previous studies have failed to clarify the causes of atherosclerosis, which appears in the very early stages of the disease and is very common in CKD patients. In addition to the traditional risk factors (age, increased blood pressure, DM, dyslipidemia, obesity, smoking habit), which were identified in the Framingham study as well as other epidemiological studies, some new uremia-related risk factors are also thought to be associated with the development of atherosclerosis in CKD patients.Citation5,Citation6
Although there have been no previous studies investigating the relationship between sodium consumption and IMT in CKD patients, three studies have investigated the relationship between sodium consumption and IMT in non-CKD cases.Citation13,Citation27,Citation28 Njoroge et al.Citation27 evaluated 258 normotensive overweight and obese adults and determined that there is a significant positive correlation between an increase in urinary sodium excretion and an increase in carotid IMT and this correlation is independent of blood pressure value, as was found in the present study.Citation27 Ferreire-Sae et al.Citation13 conducted a study in 42 hypertensive adult patients and observed that the relationship between sodium consumption and an increase in IMT as detected in a univariate analysis was not detectable in a multivariate analysis. Garcia-Ortiz et al.Citation28 determined a j-shaped relationship between sodium consumption and IMT, which is not statistically significant. The patients’ sodium consumption was measured by 24-h urinary sodium excretion in the Njoroge et al. study,Citation27 which is the study most similar to the present study, whereas it was determined by diet surveys (recall) in the other studies.Citation13,Citation28 It has been demonstrated that almost the entire daily dietary sodium intake is absorbed through the gastrointestinal system and that there is an exact balance between sodium consumption and urinary sodium excretion in a steady state.Citation29 On the other hand, it was determined that a personal dietary report-based assessment is likely to misdetect daily sodium intake by up to 15%.Citation21 Therefore, in the present study, which did not include patients with salt-losing nephropathy, 24-h urinary sodium excretion was used as the marker of daily sodium consumption.
Excessive sodium consumption has been shown to be associated with the development of hypertension and cardiovascular disease in the general population.Citation19 Therefore, the WHO specified that the maximum acceptable daily sodium intake for healthy adults is 2 g.Citation19 In the KDIGO guideline, the maximum amount of daily sodium recommended for CKD patients was 2 g, which is equal to that recommended for healthy adults.Citation23 In the present study, finding that IMT was thicker than 0.066 mm in the patients consuming more than 2 g of sodium in a day and determining that IMT is an independent determinant of urinary sodium excretion and that each 2 g (approximately 85 mmol) increase in urinary sodium excretion increases IMT by 0.031 mm, even after adjusting for all IMT-related factors, suggests that excessive sodium consumption is an important factor in the acceleration of the development of atherosclerosis in CKD patients.
Although the mechanism of vascular injury-generating and atherosclerosis-accelerating effects of excessive dietary sodium intake has not been fully explained, it was demonstrated that shear–stress, which is generated by a blood pressure-increasing effect, can trigger the development of atherosclerosis by initiating endothelial damage.Citation30 In the present study, finding that blood pressure values were significantly higher in the patients consuming more than 2 g of sodium as compared to the patients consuming a smaller amount of sodium and determining that SBP is the independent determinant of IMT, even after adjusting for all factors, suggests that shear–stress, which is developed by the hemodynamic effect, played a key role in the development of atherosclerosis in our patients.
Excessive sodium consumption has been shown to lead to atherosclerosis, independent from the blood pressure-increasing effect, and that it has a facilitating effect on the development of left ventricle hypertrophy and stroke.Citation16,Citation17 There are evidences suggesting that more than one factor is responsible for the relationship between excessive sodium consumption and direct vascular injury.Citation9–15,Citation31–36 Experimental studies demonstrated that excessive sodium consumption increases the production of reactive oxygen species (ROS).Citation9 It was determined that ROS decreases the bioavailability of NO by transforming it into peroxynitrite via the NO-scavenger effect and decreases production of NO by enhancing oxidation of tetrahydrobiopterin, a cofactor for endothelial NO synthase.Citation10 Dupont et al.Citation31 gave a diet containing 13–15 g/day of sodium to salt-resistant normotensive individuals for seven days and observed remarkable impairment in flow-mediated dilatation (FMD), despite the absence of any change in blood pressure. Jablonski et al.Citation10 determined that a local antioxidant (vitamin C) infusion, in addition to a high-salt diet improved the impaired FMD in adult patients with blood pressure changes between 130 and 159 mmHg. Demonstrating that (i) sodium experimentally enhances angiotensin (ang) converting enzyme activity at tissue level, (ii) conversion of ang I into ang II is increased in the people consuming excessive sodium, and moreover, (iii) cell proliferation-enhancing effect of ang II becomes strong in the cell cultures containing high concentration of sodium reveals that activation of RAS plays a key role in direct relationship between excessive sodium consumption and atherosclerosis.Citation32–34 On the other hand, demonstrating that sodium consumption is associated with elevation of hs-CRP in hypertensive individuals independent from blood pressure values reveals that inflammation may be another predisposing factor in the relationship between sodium intake and atherosclerosis.Citation35 It has also been demonstrated that excessive sodium consumption accelerates sodium transport into the endothelial cells by increasing the abundance of epithelial sodium channels and thereby might cause an increase in endothelial cell stiffness due to the damage in endothelial glycocalyx.Citation11,Citation12 It has been determined that excessive sodium intake leads not only to endothelial stiffness, but also to the increase in arterial stiffness, by enhancing endothelial transforming growth factor-β production and metalloproteinase-9 activity.Citation13,Citation14 Consuming even a single cup of soup containing 1.5 g of sodium has been shown to increase arterial stiffness in normotensive patients without any change in blood pressure.Citation36 The relationship between sodium consumption and IMT, found in the present study, is independent from blood pressure values, and this suggests that excessive sodium consumption might have made a contribution to the development of atherosclerosis not only by increasing blood pressure, but also by causing vascular damage.
Asaria et al.Citation37 suggested that a 15% decrease in sodium consumption in a population over 10 years would result in an 8.5 million decrease in cardio-vascular deaths. However, worldwide sodium consumption remains unacceptably high. Worldwide mean sodium consumption is estimated to be approximately 4 g per day.Citation38 In the present study, we determined that 77% of the patients have been consuming more than 2 g of sodium per day, which is the upper limit of the recommended daily amount. Other studies of CKD patients determined that their sodium consumption was similar to the general population (approximately 3–4.5 g per day) and that 60–90% of CKD patients have been consuming excessive sodium.Citation39 The most important reasons for the patients’ high sodium consumption, despite the fact that refrigerators and deep freezers have been put into use and hence the use of sodium as a preservative is no longer necessary, include an unknown amount of sodium contents of foods and increased consumption of processed foods. In the industrialized countries, home-cooked foods or adding salt at the table (using a salt shaker) accounts for only 12% of the daily sodium intake, whereas processed foods account for 77%.Citation40 Informing our patients about not having salt shakers on the table or not adding salt to a meal while cooking does not result in a salt-free diet at this point. Due to a lack of awareness on this subject, patients worldwide, unfortunately, do not understand how much sodium they consume, how they poison themselves with the sodium content of processed foods, and that they substantially increase their risk of atherosclerotic disease. Decreased blood pressure as the response to sodium restriction has been shown to be two times higher in hypertensive patients with stage 3–4 CKD as compared to the hypertensive patients without CKD.Citation41 This suggests that a decrease in daily sodium consumption in CKD patients, who are known to be more susceptible to excessive sodium intake, may lead to a greater decrease in carotid artery IMT as compared to the healthy population and the patient groups with other chronic diseases.
While evaluating the results of the present study, its observational and cross-sectional design, not including black patients, and most of its univariate correlations being weak (r < 0.300) should be taken into account. However, the fact that the relationships between nutritional parameters and clinical/laboratory data determined in the previous studies are similar (r = 0.200–0.300) to the present study strengthens the clinical significance of the relationship determined in the present study.Citation13,Citation27,Citation42
Our results reveal a relation between urinary sodium excretion and carotid artery intima-media thickness and suggest that excessive sodium consumption predisposes development of atherosclerosis in patients with chronic kidney disease.
Declaration of interest
The authors report no conflicts of interest.
References
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol. 2006;17:2034–2047
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663
- Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;9(12):S16–S23
- Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:672–681
- Sarnak MJ, Coronada BE, Greene T, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57:327–335
- Delahaye F. Should we eat less salt? Arch Cardiovasc Dis. 2013;106:324–332
- Intersalt Cooperative Research Group. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excretion. BMJ. 1988;297:319–328
- Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14
- Jablonski KL, Racine ML, Geolfos CJ, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343
- Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012;463:269–278
- Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflugers Arch. 2014;466:851–859
- Ferreira-Sae MC, Cipolli JA, Cornélio ME, et al. Sodium intake is associated with carotid artery structure alterations and plasma matrix metalloproteinase-9 upregulation in hypertensive adults. J Nutr. 2011;141:877–882
- Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol. 1999;277:H1293–H1298
- Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow-up study. Am J Med. 2006;119:275.e7–275.e14
- Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: http://dx.doi.org/10.1136/bmj.b4567
- Kupari M, Koskinen P, Virolainen J. Correlates of left ventricular mass in a population sample aged 36 to 37 years. Focus on lifestyle and salt intake. Circulation. 1994;89:1041–1050
- Perry IJ, Beevers DG. Salt intake and stroke: A possible direct effect. J Hum Hypertens. 1992;6:23–25
- World Health Organisation. Guideline: Sodium intake for adults and children. Geneva: WHO; 2012. Available at: apps.who.int/iris/bitstream/10665/77985/1/9789241504836_eng.pdf. Accessed January 26, 2015
- Ji C, Sykes L, Paul C, et al. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. 2012;32:307–315
- Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24-hour urinary excretion. Hypertension. 2014;63:238–244
- World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17(2):151–183
- Kidney disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:337–414
- Modi N, Kapoor A, Kumar S, Tewari S, Garg N, Sinha N. Utility of carotid intimal medial thickness as a screening tool for evaluation of coronary artery disease in pre-transplant end stage renal disease. J Postgrad Med. 2006;52:266–270
- Salonen JT, Salonen R. Ultrasonograhically assessed carotid morphology and risk of coronary heart disease. Arterioscler Thromb. 1991;11:1245–1249
- O’Leary D, Polak J, Kronmal R, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:12–22
- Njoroge JN, El Khoudary SR, Fried LF, Barinas-Mitchell E, Sutton-Tyrrell K. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. Am J Hypertens. 2011;24:70–76
- García-Ortiz L, Recio-Rodríguez JI, Rodríguez-Sánchez E, et al. Sodium and potassium intake present a J-shaped relationship with arterial stiffness and carotid intima-media thickness. Atherosclerosis. 2012;225(2):497–503
- Holbrook JT, Patterson KY, Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40:786–793
- Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;24:8–13
- DuPont JJ, Greaney JL, Wenner MM, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31:530–536
- Kocks MJ, Buikema H, Gschwend S, Boomsma F, de Zeeuw D, Navis G. High dietary sodium blunts affects of angiotensin-converting enzyme inhibition on vascular angiotensin I-to-angiotensin II conversion in rats. J Cardiovasc Pharmacol. 2003;42:601–606
- Boddi M, Poggesi L, Coppo M, et al. Human vascular renin-angiotensin system and its functional changes in relation to different sodium intakes. Hypertension. 1998;31:836–842
- Liu G, Hitomi H, Rahman A, et al. High sodium augments angiotensin II-induced vascular smooth muscle cell proliferation through the ERK 1/2-dependent pathway. Hypertens Res. 2014;37:13–18
- Yilmaz R, Akoglu H, Altun B, Yildirim T, Arici M, Erdem Y. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr. 2012;66:1214–1218
- Dickinson KM, Clifton PM, Burrell LM, Barrett PH, Keogh JB. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis. 2014;232:211–216
- Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: Health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053
- Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38(3):791–813
- Krikken JA, Laverman GD, Navis G. Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:531–538
- Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr. 1991;10:383–393
- McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–2103
- Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65:1179S–1186S