Abstract
Sulfasalazine is widely used for inflammatory-mediated disorders in human. Renal damage is a serious adverse effect accompanied sulfasalazine administration. No specific therapeutic option is available against this complication so far. Oxidative stress seems to play a role in sulfasalazine-induced renal injury. Current investigation was designed to evaluate the effect of N-acetyl cysteine (NAC) and dithiothreitol (DTT) as thiol reductants against sulfasalazine-induced renal injury in rats. Oral administration of sulfasalazine (600 mg/kg for 14 consecutive days) caused renal injury as judged by increase in serum level of creatinine and blood urea nitrogen. Furthermore, the level of reactive oxygen species and lipid peroxidation were raised in kidney tissue after sulfasalazine administration. Additionally, it was also found that renal glutathione reservoirs were significantly depleted in sulfasalazine-treated animals. Histopathological examination of kidney endorsed organ injury in drug-treated rats. Daily intraperitoneal administration of NAC (250 and 500 mg/kg/day) and/or DTT (15 and 30 mg/kg/day) effectively alleviated renal damage induced by sulfasalazine. Data suggested that thiol reductants could serve as potential protective agents with therapeutic capabilities against sulfasalazine adverse effect toward kidney.
Introduction
Renal injury is accompanied by administrations of several drugs.Citation1 Sulfasalazine is widely administered against inflammatory-mediated diseases in human.Citation2 On the other hand, some investigations indicate decrease in renal function in sulfasalazine-treated patients.Citation3,Citation4 Sulfasalazine-induced renal injury has typically been presented as interstitial nephritis, glomerulonephritis, nephritic syndrome and acute renal failure.Citation5–7 Sulfasalazine-induced renal injury, might be potentially irreversible.Citation8,Citation9 No specific therapeutic agent has been developed against sulfasalazine-induced kidney injury so far.
Although there is no precise mechanism known to be responsible for sulfasalazine-induced renal injury, some researches indicated the role of reactive metabolites, reactive oxygen species (ROS) and oxidative stress in the organ injury induced by this drug.Citation10,Citation11 Moreover, the oxidative stress has also been reported to be involved in other side effects of sulfasalazine.Citation10
N-acetyl cysteine (NAC) and dithiothreitol (DTT) are robust thiol reducing agents which their cytoprotective properties have been established in different experiments.Citation12 Due to the involvement of oxidative stress in sulfasalazine renal injury,Citation10,Citation11 it is expected that thiol reducing agents offer protective properties against this complication.
Material and methods
Animals
Male Sprague-Dawley rats (n = 36) weighing 250–300 g were obtained from the Laboratory Animal Breeding Center, Shiraz University of Medical Sciences, Shiraz, Iran. Animals were housed in an environmental temperature of 22 ± 2 °C with a 50–60% of relative humidity. Rats had free access to water and a normal chow diet. All the experiments were performed in conformity with the guidance for care and use of experimental animals approved by an ethic committee in Shiraz University of Medical Sciences, Shiraz, Iran.
Experimental setup
Animals were randomly divided into six groups containing six rats in each. Rats were treated as follows: (1) control (vehicle-treated group), (2) sulfasalazine (600 mg/kg/day, oral); (3) sulfasalazine (600 mg/kg/day, oral) + NAC (250 mg/kg/day, i.p); (4) sulfasalazine (600 mg/kg/day, oral) + NAC (500 mg/kg/day, i.p); (5) sulfasalazine (600 mg/kg/day, oral) + DTT (15 mg/kg/day, i.p) and (6) sulfasalazine (600 mg/kg/day, oral) + DTT (30 mg/kg/day, i.p).
It has been previously reported that a dose of 600 mg/kg/day of sulfasalazine for 14 consecutive days caused marked renal injury in rats.Citation10 None of the investigated thiol reductants affected markers of organ injury assessed in current investigation, when they were administered alone at the above-mentioned doses. At the end of experiments, animals were anesthetized (sodium thiopental, 80 mg/kg, i.p) and their blood and kidney were collected.
Serum biochemistry and organ histopathology
A Mindray BS-200® auto analyzer (Mindray®, Shenzhen, China) and standard kits (Pars Azmun®, Tehran, Iran) were employed to assess serum creatinine (Cr) and blood urea nitrogen (BUN).Citation13 For histopathological assessments, samples of kidney tissue were fixed in buffered formalin solution (0.4% sodium phosphate monobasic, NaH2PO4, 0.64% sodium phosphate dibasic, Na2HPO4, and 10% formaldehyde in distilled water). Paraffin-embedded sections of tissue were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing.Citation14
Reactive oxygen species formation
ROS in kidney tissue was estimated by a method described by Gupta et al.,Citation15 with some modifications. Kidney samples (200 mg) were homogenized in ice-cold Tris-HCl buffer (40 mM, pH = 7.4) (1:10 w/v). Then, samples of tissue homogenate (100 μL) were mixed with Tris-HCl buffer (1 mL) and 5 μL of 2′,7′-dichlorofluorescein diacetate (10 μM) (Sigma-Aldrich, St. Louis, MO). The mixture was incubated for 30 min in 37 °C (Gyromax™ incubator shaker). Finally, the fluorescence intensity of the samples was assessed using a FLUOstar Omega® multifunctional microplate reader (λexcitation = 485 nm and λemission = 525 nm) (BMG Labtech, Germany).Citation15,Citation16
Measurement of lipid peroxidation
Thiobarbituric acid reactive substances were assessed in kidney as an index of lipid peroxidation. The reaction mixture was consisted of thiobarbituric acid (0.375%, w/v) (Sigma-Aldrich, St. Louis, MO), phosphoric acid (1% w/v, pH = 2) (Merck, Darmstadt, Germany), and 500 μM of tissue homogenate (10% w/v in KCl, 1.15%). The mixture was shaken well and heated in boiling water (100 °C) for 45 min. After the incubation period, 2 mL of n-butanol (Merck, Darmstadt, Germany) was added and vigorously shaken. Samples were centrifuged (3000g for 5 min) and the absorbance of developed color in n-butanol phase was measured at 532 nm using an Ultrospec 2000®UV spectrophotometer (Pharmacia Biotech, Sweden).Citation17
Kidney glutathione content
Tissue samples (200 mg) were homogenized in 8 mL of ice-cooled EDTA (20 mM) (Merck, Darmstadt, Germany). Five milliliter of the prepared homogenate was mixed with 4 mL of distilled water and 1 mL of trichloroacetic acid (50% w/v). The mixture was shaken and centrifuged (10,000 g, 4 °C, 25 min). Then, 2 mL of the supernatant was mixed with 4 mL of Tris buffer (pH = 8.9), and 100 μl of Ellman’s reagent (DTNB, 0.01 M in methanol). The absorbance of the developed color was measured in 412 nm using an Ultrospec 2000® UV spectrophotometer (Pharmacia Biotech, Sweden).Citation13
Statistical analysis
Data are given as the Mean ± SEM. Data comparison was performed by the one-way analysis of variance with Tukey’s post hoc multiple comparison test. Differences were considered statistically significant when p < 0.05.
Results
Sulfasalazine administration (600 mg/kg/day for 14 days) caused a marked renal impairment as judged by the elevated level of serum BUN and Cr (). Moreover, it was found that sulfasalazine administration caused a significant increase in tissue level of ROS in rats’ kidney (). ROS formation in kidney tissue was accompanied by consequent lipid peroxidation and decrease in tissue glutathione (GSH) content ().
Table 1. Markers of sulfasalazine-induced renal damage and the role of thiol-reductants administration.
Histopathological examination of renal tissue specimens revealed sulfasalazine-induced kidney injury which was detected as interstitial inflammation, tubular atrophy, focal necrosis and vascular congestion ().
Figure 1. Effects of thiol reducing agents administration on kidney histopathological changes in sulfasalazine-treated animals. Rats received sulfasalazine (600 mg/kg/day, PO) for 14 consecutive days alone and/or in combination with intraperitoneal administration of thiol-reductants. (A) Photomicrograph of kidney section taken from control (vehicle-treated) rats, representing normal tubules. (B) Sulfasalazine-treated rats, showing marked tubular atrophy, focal necrosis, interstitial inflammation, and vascular congestion. (C) and (D) Animals received sulfasalazine and NAC (250 and 500 mg/kg, respectively). Tubular atrophy is detected in these groups, but there is no sign of tubular necrosis in animals treated with 500 mg/kg of NAC. (E) Rats were treated with sulfasalazine and DTT (15 mg/kg). (F) Animals received sulfasalazine and DTT (30 mg/kg). Sulfasalazine-induced focal necrosis was absent in this group.
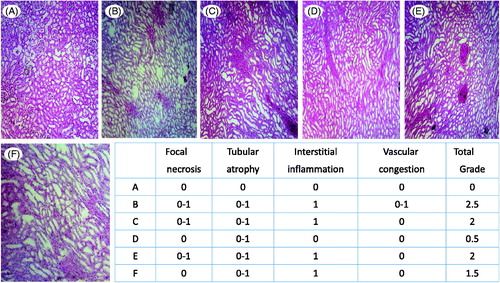
NAC (250 and 500 mg/kg, i.p., for 14 consecutive days) and DTT (30 mg/kg, i.p, for 14 consecutive days) significantly diminished elevation in serum BUN and Cr (). Kidney ROS level and lipid peroxidation were also significantly lower when NAC and/or DTT was administered to sulfasalazine-challenged rats (). Moreover, it was found that NAC and DTT prevented kidney GSH depletion in sulfasalazine-treated rats (). Finally, sulfasalazine-induced kidney histopathological changes were mitigated when animals received NAC and/or DTT ().
Discussion
Current investigation aimed to evaluate the effect of thiol reductants against sulfasalazine-induced renal injury in rats. NAC (250 and 500 mg/kg, i.p) and DTT (15 and 30 mg/kg, i.p) administration effectively attenuated sulfasalazine-induced renal injury which was manifested by preventing the elevation in serum Cr and BUN as markers of renal impairment. Moreover, NAC and DTT mitigated sulfasalazine-induced ROS formation, lipid peroxidation, GSH depletion and finally tissue lesions in rat kidney.
We found that kidney ROS formation as an indicator of oxidative stress was significantly increased in sulfasalazine-treated animals. Multiple intracellular targets are subject to affect by oxidative stress, including proteins, lipids and nucleotides.Citation18 It has been reported that cellular defense mechanisms are impaired in sulfasalazine-treated animals.Citation10,Citation11 The impairment in cellular defense mechanisms induced by sulfasalazine, might led to generation of excess ROS production and finally organ dysfunction.
Sulfasalazine is degraded to mesalazine and sulfapyridine by bacterial azo reductases in large intestine. Sulfapyridine is almost completely absorbed compared with about 20–30% absorption for mesalazine. Approximately 10–30% of sulfasalazine is also absorbed unchanged from the small intestine.Citation2 It is not clear whether the whole molecule of sulfasalazine and/or its intestinal metabolites are responsible for the renal injury induced by this drug. Some investigations mentioned the role of mesalazine in the nephrotoxic reactions after sulfasalazine administration. Although mesalazine is recognized as a chemical which might induce renal injury,Citation2,Citation5,Citation9 the administered dose of mesalazine needed to induce renal injury is much higher than a mesalazine dose which a patient will receive during sulfasalazine therapy. Hence, other mechanisms and the whole molecule of sulfasalazine might be involved in the renal injury induced by this drug. Sulfapyridine as the other metabolite of sulfasalazine is a chemical with sulfonamide structure. Sulfonamides are well known for their adverse effects toward kidney.Citation19 Some investigations reported the potential role of sulfapyridine in sulfasalazine-induced renal injury.Citation20 Despite the cause of renal injury accompanied sulfasalazine administration, it seems that oxidative stress and its consequent events play a major role in this complication. In current investigation we found that thiol reducing agents counteracted the deleterious effects of sulfasalazine on the renal function as ROS formation and kidney histopathological lesions were mitigated by NAC and DTT.
Therapeutic doses of sulfasalazine administered to human are between 3 and 6 g/day. As a high dose of the drug is administered in a chronic manner, it might cause cellular defense mechanisms impairment and continuous oxidative stress in patients’ kidney. Thiol reducing agents such as NAC might be capable of to be administered as a supplement agent in sulfasalazine-treated patients. The proposed mechanism(s) of sulfasalazine-induced renal injury and the potential therapeutic effects of thiol reductants are summarized in .
Figure 2. Sulfasalazine-induced renal damage and the proposed mechanisms of reno-protection provided by thiol-reductants.
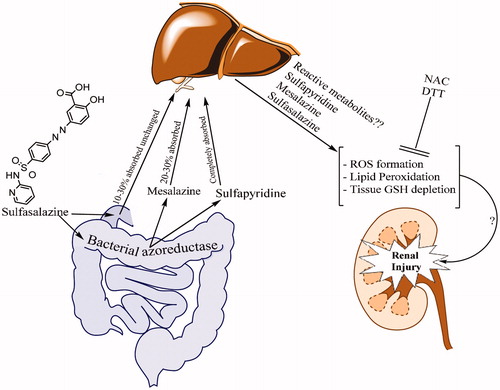
Interestingly, the antioxidant and anti-inflammatory properties of sulfasalazine in kidney are also reported in previous investigations.Citation21 The reported renoprotective dose of sulfasalazine is lower (100 mg/kg for 7 days) than the nephrotoxic dose which is applied in current investigation. Hence, we might be able to conclude that sulfasalazine serves as a renoprotective agent in lower doses which reduce inflammation and cytotoxic cytokines probably by inhibiting nuclear factor kB (NF-kB) in kidney. In current investigation, we found that higher doses of sulfasalazine (600 mg/kg for 14 days) caused oxidative stress in kidney as revealed by increase in ROS, lipid peroxidation and tissue GSH depletion. As sulfasalazine (but not its metabolites like sulfapyridine and 5-aminosalisilic acid) is a very strong and specific NF-kB inhibitor,Citation22 sulfasalazine metabolites might contribute in the nephrotoxic properties of this drug in higher doses.
In conclusion, we suggest that thiol reducing agents have a protective role against sulfasalazine-induced renal injury probably by attenuating oxidative stress and restoring GSH level in kidney. Further future investigations are needed to reveal the precise mechanism of sulfasalazine-induced renal injury and endorse the renoprotective properties of thiol reductants.
Acknowledgements
The technical facilities providing of Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences is gratefully acknowledged.
Declaration of interest
This investigation was financially supported by the office of the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences (#93-01-36–7612). Authors do not have conflicts of interest.
References
- Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–468
- Plosker GL, Croom KF. Sulfasalazine: A review of its use in the management of rheumatoid arthritis. Drugs. 2012;65:1825–1849
- Dwarakanath AD, Michael J, Allan RN. Sulphasalazine induced renal failure. Gut. 1992;33:1006–1007
- Molnár T, Farkas K, Nagy F, et al. Sulfasalazine-induced nephrotic syndrome in a patient with ulcerative colitis. Inflamm Bowel Dis. 2010;16:552–553
- Birketvedt GS, Berg KJ, Fausa O, Florholmen J. Glomerular and tubular renal functions after long-term medication of sulphasalazine, olsalazine, and mesalazine in patients with ulcerative colitis. Inflamm Bowel Dis. 2000;6:275–279
- Barbour VM, Williams PF. Nephrotic syndrome associated with sulphasalazine. BMJ 1990;301:818–819
- Das KM, Eastwood MA, McManus JPA, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. New Engl J Med. 1973;289:491–495
- Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514
- Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis. 2007;13:629–638
- Alonso V, Linares V, Bellés M, et al. Sulfasalazine induced oxidative stress: A possible mechanism of male infertility. Reprod Toxicol. 2009;27:35–40
- Linares V, Alonso V, Domingo JL. Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert Opin Drug Saf. 2011;10:253–263
- Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2001;36:151–180
- Heidari R, Jamshidzadeh A, Keshavarz N, Azarpira N. Mitigation of methimazole-induced hepatic injury by taurine in mice. Sci Pharm. 2014;83:143–158
- Moezi L, Heidari R, Amirghofran Z, et al. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: The role of nitric oxide and IL-1β. Pharmacol Rep. 2013;65:134–143
- Gupta R, Dubey DK, Kannan GM, Flora SJS. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int. 2007;31:44–56
- Socci DJ, Bjugstad KB, Jones HC, et al. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol. 1999;155:109–117
- Heidari R, Niknahad H, Jamshidzadeh A, et al. Carbonyl traps as potential protective agents against methimazole-induced liver injury. J Biochem Mol Toxicol. 2014;29:173–181
- Avery S. Molecular targets of oxidative stress. Biochem J. 2011;434:201–210
- Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555–565
- Russinko PJ, Agarwal S, Choi MJ, Kelty PJ. Obstructive nephropathy secondary to sulfasalazine calculi. Urology. 2003;62:748–750
- Demirbilek S, Emre MH, Aydın EN, et al. Sulfasalazine reduces inflammatory renal injury in unilateral ureteral obstruction. Pediatr Nephrol. 2007;22:804–812
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174
