Abstract
Background The efficacy of tonsillectomy in immunoglobulin A nephropathy (IgAN) remains controversial. The aim of the study was to conduct a randomized controlled trial to evaluate the effect of tonsillectomy in patients with IgAN. Methods We randomly selected 98 patients with biopsy-proven IgA nephropathy and randomly allocated to receive tonsillectomy combined with drug therapy (Group A) or drug therapy alone (Group B). The participating patients were entered into a 4-year single-center study. Remission and relapse rate were calculated for hematuria and proteinuria using the Kaplan–Meier method. Results No differences were found between the two groups in their baseline clinical and histological characteristics. Patients with tonsillectomy exhibited considerable improvement in the following aspects compared to those patients who did not undergo tonsillectomy: time to reach first remission (3.1 vs. 24.9 months, p < 0.001) for hematuria and (2.5 vs. 26.1 months, p < 0.001) for proteinuria, cumulative remission rate (91.8% vs. 46.9%, p < 0.001 by log-rank test) for hematuria and (95.9% vs. 51.0%, p < 0.001) for proteinuria, the duration of first remission (26.5 vs. 11.8 months, p = 0.0047) for hematuria and (23.5 vs. 10.5 months, p = 0.0012) for proteinuria, as well as lower relapse rate for hematuria and proteinuria in Group A. Conclusion Our clinical data demonstrated that tonsillectomy could be beneficial for IgAN patients, particularly by contributing to faster and longer remission, as well as reducing the frequency of possible future relapses.
Introduction
Immunoglobulin A nephropathy (IgAN) is recognized as the most common immune complex related to the cause of glomerulonephritis worldwide.Citation1 The single diagnostic feature of IgAN is the finding of immune deposits predominantly containing polymeric IgA in the glomerular mesangium on renal biopsy.Citation2 Approximately 20–50% of IgAN patients would develop end-stage renal disease (ESRD) over the course of roughly 20 years,Citation3 thereby rekindling interest in the development of an effective, targeted therapy.
The classical clinical picture in IgAN of recurrent episodes of visible hematuria coinciding with upper respiratory or intestinal tract infections indicating that infections induced abnormal mucosal immunity plays an important role in the pathogenesis of IgAN.Citation4
Among these lymphoid tissues, tonsil is the largest mucosal immune organ in the human body.Citation5 Tonsils consist of palatine tonsil, tubal tonsil, adenoid tonsil, and lingual tonsil, which jointly form a structure called Waldeyer’s tonsillar ring,Citation6 the portal of the aerodigestive tract. According to some previous studies, tonsillectomy, combined with steroid pulse therapy, could significantly improve the urine test results and stabilize the renal function of IgA nephropathy patients during a long-term period.Citation5,Citation7 In addition, in 1-year observation, tonsillectomy plus steroid pulse therapy could reduce the frequency of relapse based on the investigation of Ohya et al.Citation8 However, this was a retrospective cohort study.
Herein, we report the first randomized controlled clinical study that provided strong evidence for prolonged remission and fewer relapses frequency among tonsillectomized IgAN patients. Since IgAN can lead to repeated renal inflammations and relapses, we envision that the results of our clinical study would serve to demonstrate the efficacy of tonsillectomy in IgAN.
Materials and methods
Patient selection and randomization
This study was conducted between 1 June 2009 and 30 November 2010 at the Second Xiangya Hospital of Central South University. Our institution routinely performed tonsillectomy combined with drug therapy to treat IgAN. This study was conducted in accordance with the guidelines proposed in the Declaration of Helsinki. All of the adults gave their written informed consent.
The inclusion criteria were established primarily according to the previous trials,Citation9,Citation10 and were biopsy-proven IgAN, an age ranging from 18 to 69 years, urinary protein excretion ranging from 1.0 to 3.5 g/day, serum creatinine of ≤1.5 mg/dL, and systolic and diastolic blood pressures of <140 and <90 mmHg, respectively, regardless of the use or non-use of antihypertensive drugs. Exclusion criteria were nephrotic syndrome, serum creatinine of >1.5 mg/dL, recent treatment with corticosteroids and/or immunosuppressive agents, and contraindications for general anesthesia and/or tonsillectomy as assessed by otolaryngologists. Patients with systemic diseases, such as systemic lupus erythematosus, Henoch–Schönlein purpura, rheumatoid arthritis, and chronic liver diseases were also excluded from this study. The participants were randomly assigned to receiving tonsillectomy combined with drug therapy (Group A) or drug therapy alone (Group B). The randomization was performed through a preprinted randomization table.
Data collection and evaluation
During the trial, the patients were examined every other month for blood pressure, urinary protein excretion, serum creatinine and urinary sediment with red blood cells (RBC). Other data including the incidence of adverse effects and the prescription of additional drugs were also obtained. Urinary protein at baseline was represented by a mean value from three consecutive data points prior to the treatment (i.e., tonsillectomy in Group A, first tripterygium glycosides treatment in Group B). Estimated glomerular filtration rate (GFR) (mL/min/1.73m2) was calculated using the abbreviated MDRD formula modified for Chinese subjectsCitation11: GFR = 186 × [SCr×0.011] – 1.154 × [age] – 0.203 × [0.742 (if female)] × 1.233 (if Chinese), where SCr is serum creatinine expressed as micromolar. Urine samples were collected at predetermined time points and analyzed via urinalysis for diagnosis of hematuria and/or proteinuria. The presence of five or more RBC per high field under optical microscope was considered as direct proof of hematuria. On the other hand, proteinuria was indicated by 24-h urine total protein exceeding 300 mg.
All tissue specimens used in the study were obtained from the participating patients by percutaneous needle biopsy. The tissue samples were analyzed evaluated by light microscopy for a variety of pathological features, including global sclerosis, segmental sclerosis, crescent, balloon adhesion, peripheral capsule fibrosis, mesangial proliferation, and tubular atrophy/interstitial fibrosis. Histological grade was assessed by the classification proposed by the Special IgAN Study Group in 2004.Citation12
Medications protocol
Patients of group A received both tonsillectomy and drug therapy while group B with drug therapy alone. We followed the drug treatment protocol as previously reportedCitation13: from the time following up patients received oral tripterygium glycosides, an extract from a medicinal plant and benefit for IgANCitation14 (20 mg/thrice daily) therapy for 2 months and gradually reduced the dose according to the urinary improvement. We tapered the tripterygium glycosides dose gradually by 10 mg per 2 weeks down to a dosage of 20 mg/day which lasted for 12 months.
At the start of following up, if the urinary protein was greater than 1 g/24 h, the oral prednisone (30 mg/day) was added and lasted for a period of 2–3 months, then gradually withdrawn and lasted at the dose of 10 mg/day for 12 months. Among them, 13 patients of group A and 12 of group B were treated with prednisolone, and there was no difference between numbers of two groups who underwent prednisolone therapy (p < 0.05). When needed, the patients in both groups were given antihypertensive drugs to control blood pressure to <125/75 mmHg during the trial period. RAS inhibitors (valsartan 80 mg once daily) were the primary antihypertensive drugs recommended during the study.
Assessment of clinical (hematuria and proteinuria) remission and relapse
In these patients, the disappearance of proteinuria was defined as levels of <0.3 g/day. Disappearance of hematuria was defined as a number of RBC in urinary sediments of <5 per high power field. Clinical remission (CR) was defined as the disappearance of both proteinuria and hematuria. Relapse from CR was defined as the reappearance of urinary abnormalities (i.e., either hematuria or proteinuria or both).
Duration time of first remission was defined as the elapsed time between the first remission and the next observed recurrence of either urinary protein or urinary RBC.
All participating IgAN patients are graded on the recovery level by the number of relapses they were observed to undergo per 6 months, resulting in the three remission categories shown below:
None relapses: no more than one relapse of hematuria or proteinuria was observed per every 6 months.
Relapse with low frequency: no more than two relapses of hematuria or proteinuria were observed per every 6 months.
Relapse with high frequency: more than two relapses of hematuria or proteinuria were observed per every 6 months.
Statistical analysis
The data were expressed in the form of either mean ± SD or number (%). The demographic and the clinical characteristics of the patients were described and analyzed using standard descriptive statistics. For comparing the parameters between the two groups, the unpaired t-test and non-parametric Wilcoxon rank-sum test were used for normally and non-normally distributed variables, respectively. The difference in frequency between the two groups was evaluated using Pearson’s chi-square test. The remission rate and relapse rate were calculated for hematuria and proteinuria using the Kaplan–Meier method. A value of p < 0.05 was considered significant. Statistical analyses were performed with SPSS 17.0 and Graphpad Prim 5.0.
Results
Clinical characteristic at the time of renal biopsy
A total of 98 patients with biopsy-proven IgAN and who fulfilled the trial entry criteria were randomly allocated to receive tonsillectomy with drug therapy (Group A) or drug therapy alone (Group B). The baseline demographic, biochemical, and histopathological parameters were comparable between the 2 groups. There were no differences between the 2 groups in the main baseline characteristics ().
Table 1. Clinical characteristics of the initial cohort at renal biopsy classified by tonsillectomy.
The histological grading of all renal biopsy samples was performed in accordance with the Oxford classification.Citation15 Areas of global sclerosis, cellular crescents, and vascular damage, such as vessel hyalinization or vascular wall thickening (all or none) were also counted. The renal pathology characteristics of the patients are summarized in . No significant difference in renal pathology between the two groups.
Table 2. Baseline histological characteristics.
Effect of tonsillectomy on serum IgA and IgA/C3 ratio
The IgA/C3 ratio has been regarded as a useful marker for predicting histological severity of kidney lesions in patients with IgAN.Citation16 So we compared the serum IgA and IgA/C3 ratio before and after the tonsillectomy in patients with IgAN. As shows, serum IgA concentration was 2.95 ± 1.23 mmol/L in Group A and 3.00 ± 1.45 mmol/L in Group B at the initial of the study (p > 0.05), patients that received tonsillectomy experienced significantly decrease in IgA concentration after month 6 compared to Group B. The results of IgA/C3 ratio in month 6 were consistent with those above (Group A 2.13 ± 0.39 versus Group B 2.69 ± 0.50; p < 0.05).
Table 3. Mean serum immunoglobulin A (IgA) and complement factor 3 (C3) levels and the IgA/C3 ratio in patients with IgA nephropathy at month 6.
For the dysmorphic RBC, according to the results of the urinary sediment artificial RBC count, the urinary RBC (×104/mL) decreased from 13.49 ± 9.37 to 3.58 ± 1.19 in Group A and 13.54 ± 9.37 to 7.58 ± 1.19 in Group B at month 6. It seems that tonsillectomy has a larger tendency to attenuate the hematuria.
Clinical remission
The remission rate of hematuria was 91.8% (45/49) in Group A and 46.9% (23/49) in Group B. As shown in , the adjusted cumulative remission rate was higher among patients in Group A compared to those in Group B (log-rank test, p < 0.001). The median time to reach first remission for Group A and Group B was calculated to be 3.10 (0.29) and 24.90 (10.07) months, respectively.
Figure 1. Efficacy of tonsillectomy in urinalysis remission with IgAN patients: cumulative remission rate (91.8% vs. 46.9%, p < 0.001 by log-rank test) for hematuria (A) and (95.9% vs. 51.0%, p < 0.001) for proteinuria (B). The remission rate was calculated for hematuria and proteinuria using the Kaplan–Meier method.
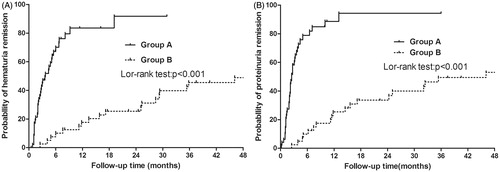
During the final clinical observation, remission of proteinuria was observed among a total number of 95.9% (47/49) patients in Group A and 51.0% (25/49) in Group B, leading to a statistically significant difference between the two (, log-rank test, p < 0.001). The median time to reach first remission for patients of Group A and of Group B was 2.50 (0.26) months and 26.10 (11.86) months, respectively.
Clinical relapse
Remission of hematuria was observed in a total number of 72 patients, among which 45 subsequently developed relapse during the study. The cumulative relapse rate of hematuria was 55.1% (27 out of 49) for Group A and 78.2% (18 out of 23) for Group B (log-rank test, p = 0.0047). As it was shown in , the follow-up time meant the duration from initiation of treatment to relapse. The median duration time of first remission for Group A and for Group B was 26.50 (5.81) months and 11.80 (2.08) months, respectively ().
Figure 2. Efficacy of tonsillectomy in urinalysis relapse with IgAN patients: the duration of first remission (26.5 vs. 11.8 months, p = 0.0047) for hematuria (A) and (23.5 vs. 10.5 months, p = 0.0012) for proteinuria (B), as well as lower relapse rate for hematuria and proteinuria in Group A. The relapse rate was calculated for hematuria and proteinuria using the Kaplan–Meier method.
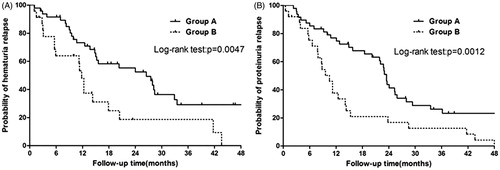
Remission of proteinuria was observed in a total number of 74 patients, among which 56 subsequently developed relapse during the study. The calculated relapse rate of proteinuria was 68.8% (33 out of 48) for Group A and 100% (25 out of 25) for Group B (log-rank test, p = 0.0012). The median duration time of first remission for Group A and for Group B was calculated to be 23.50 (5.81) months and 10.50 (2.08) months, respectively ().
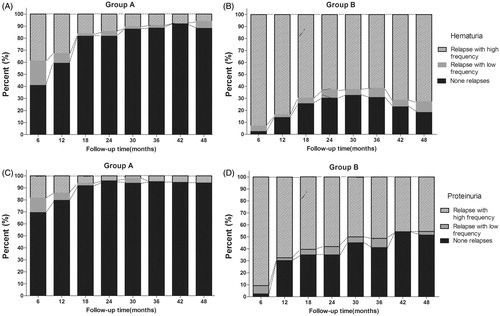
Frequency of relapse
The percentage of patients in Group A with None relapses of hematuria rose rapidly during the first 18 months following the tonsillectomy and continued to increase until eventually stabilizing at around 90% (). In contrast, the percentage of None relapses cases in Group B did not surpass 50% at any point during the study and even appeared to exhibit a mild but noticeable decline after 36 months (). Similarly, the percentage of patients with None relapses of proteinuria in Group A increased to 69.4% at 6 months after tonsillectomy (), whereas only 54.3% of Group B patients achieved None relapse at 42 months (). These results indicated a strong correlation between the performance of tonsillectomy and an increased percentage of patients having fewer relapses.
Discussion
Although several investigators already reported the beneficial effect of tonsillectomy on CR and prevention of relapse of IgAN, most of the studies were retrospective cohort studies; we were unable to adjust uniformly for potential confounding variables. So we performed a randomized controlled trial of tonsillectomy to evaluate its effect on IgAN. Our data clearly shown that tonsillectomy combined with drug therapy had additional benefit both in the achievement of CR and reducing relapse.
IgAN is not a benign disease; according to a retrospective cohort analysis evaluated clinical and histological findings in 1012 patients diagnosed with IgAN, with about 50% of patients progressing to ESRD within 30 years despite treatment, this study also shown that tonsillectomy plus steroids dramatically improved renal outcome.Citation17 Although the recent Kidney Disease: Improving Global Outcomes clinical guideline for glomerulonephritis suggests that tonsillectomy not be performed for IgAN.Citation18
As we know, the galactose-deficient IgA1 (GdIgA1), the attributer to IgAN, has a deficiency in the O-glycan located at the Hinge region of IgA1.Citation19 GdIgA1 combined with its antibodies form circulating immune complex (CIC), which eventually deposit in kidney and cause the renal deterioration.Citation20
Until recently, the question whether tonsillectomy has a role in the treatment of patients with IgAN remains controversial. Some researchers argued that aberrantly glycosylated IgA1 can also be seen in circulation in normal subjects following an immune response triggered by exposure to mucosal antigens such as food, bacteria or viruses. Thus, other factors, including genetic predisposition, are likely to influence the pathogenesis of IgAN,Citation21 so they suggested that the therapeutic effect of tonsillectomy in IgAN is limited.Citation22 In 2014, a multicenter randomized controlled trial conducted in Japan revealed that tonsillectomy combined with steroid pulse therapy has no beneficial effect over steroid pulses alone to attenuate hematuria and to increase the incidence of CR. Even to the antiproteinuric effect, the difference was marginal.Citation10 However, other data support the opposite opinion. IgA1 is predominantly secreted from nasal mucus, tears, saliva, and milk, taking up to 70–90% of the total IgA in human body. Nakata et al. found that serum GdIgA1 levels decreased by 59% after tonsillectomy, thus indicating that the palatine tonsils are probably a major sites of GdIgA1-producing cells.Citation23
In fact, Japanese Society of Nephrology clinical guidelines for IgAN suggested tonsillectomy plus steroid pulse therapy to achieve early CR in 2011.Citation24 Tonsillectomy may delay the progression of IgAN especially in patients with macrohematuria.Citation25 A meta-analysis involved 14 prospective and retrospective studies, and got a conclusion that as adjunct or independent therapy, tonsillectomy may induce CR and reduce the rates of ESRD in patients with IgAN.Citation18 What is more, our previously studies also shown that tonsillectomy may be a feasible treatment method for IgAN patients with chronic tonsillitis by increasing CD4 + CD25 + Treg cells, leading to clinical improvement.Citation13,Citation26 However, no one revealed the mechanism of tonsillectomy involved in IgAN treatment.
We consider that after the tonsillar infection/other antigens stimulation in IgAN patients, the over-secretion of IgA1/GdIgA1 from tonsil results in the deposition of IgA1 in glomeruli and renal damage.Citation27,Citation28 This process is dynamic, repeating, and discontinuous. When the same antigen stimulation is attenuated or disappeared, the secretion of IgA1/GdIgA1 declines, vanishes, or even recovers to its normal range. At this time, the deposited IgA1/GdIgA1 in glomeruli diminish or wane. We guess tonsils infections can be regarded as “trigger point”. In mucosal immune system, the trigger point is the stimulation of infecting pathogens or any other antigens, the CIC containing Gd-IgA1 is the bullet, and the target is kidney. So, tonsillectomy may in part cut off the “trigger point” of IgAN.
Limitations of this study were as follows. First, it was performed at a single medical center. Second, the patients have just recently completed 48 months of follow-up; therefore, a longer follow-up period is required. Furthermore, a homogeneous study population with an adequate number of patients should be enrolled.
In conclusion, our study clearly shows that tonsillectomy has a remarkable impact on both remission and prevention of relapse of hematuria and/or proteinuria in IgAN patients. Treatment strategies leading to CR and decreasing the relapse frequency should be encouraged for patients with IgAN to terminate the progressive deterioration of kidney. Tonsillectomy can contribute to the preservation of renal function in patients with IgAN patients.
Declaration of interest
The authors have no conflict of interest to disclose.
This work was supported by a research grant (81373227, 81400721, 81170663, and 81470947) from the National Natural Science Foundation of China. It was also supported by Department of Nephrology, The National Key Clinical Specialty Construction Project of China and the National Key Technology R&D Program (No. 2011BAI10B08 and 2011BAI10B03) and a research fund from Health and Family Planning Commission of Hunan province (132015-023).
References
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–2414.
- Lin M, Du L, Brandtzaeg P, Pan-Hammarstrom Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2014;7(3):511–520.
- Appel GB, Waldman M. The IgA nephropathy treatment dilemma. Kidney Int. 2006;69(11):1939–1944.
- Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10(8):445–454.
- Xie Y, Chen X, Nishi S, Narita I, Gejyo F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004;65(4):1135–1144.
- Masieri S, Trabattoni D, Incorvaia C, et al. A role for Waldeyer’s ring in immunological response to allergens. Curr Med Res Opin. 2014;30(2):203–205.
- Yamamoto Y, Hiki Y, Nakai S, et al. Comparison of effective impact among tonsillectomy alone, tonsillectomy combined with oral steroid and with steroid pulse therapy on long-term outcome of immunoglobulin A nephropathy. Clin Exp Nephrol. 2013;17(2):218–224.
- Ohya M, Otani H, Minami Y, et al. Tonsillectomy with steroid pulse therapy has more effect on the relapse rate than steroid pulse monotherapy in IgA nephropathy patients. Clin Nephrol. 2013;80(1):47–52.
- Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15(1):157–163.
- Kawamura T, Yoshimura M, Miyazaki Y, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29(8):1546–1553.
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944.
- Sato M, Hotta O, Tomioka S, et al. Cohort study of advanced IgA nephropathy: Efficacy and limitations of corticosteroids with tonsillectomy. Nephron Clin Pract. 2003;93(4):c137–c145.
- Liu Y, Liu H, Tu X, et al. Study of tonsillectomy for IgA nephropathy patients: Short- and longer-term observation. Int Urol Nephrol. 2014;46(6):1153–1159.
- Chen YZ, Gao Q, Zhao XZ, et al. Meta-analysis of Tripterygium wilfordii Hook F in the immunosuppressive treatment of IgA nephropathy. Intern Med. 2010;49(19):2049–2055.
- Peters CD, Ring T. Validation of the Oxford classification of IgA nephropathy: Valid or invalid? Kidney Int. 2015;87(3):661–662.
- Zhang J, Wang C, Tang Y, et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton). 2013;18(2):125–131.
- Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9(3):e91756.
- Liu LL, Wang LN, Jiang Y, et al. Tonsillectomy for IgA nephropathy: A meta-analysis. Am J Kidney Dis. 2015;65(1):80–87.
- Mestecky J, Raska M, Julian BA, et al. IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240.
- Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28(1):78–87.
- Gharavi AG, Kiryluk K, Choi M, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43(4):321–327.
- Zand L, Fervenza FC. Does tonsillectomy have a role in the treatment of patients with immunoglobulin A nephropathy? Nephrol Dial Transplant. 2014;29(8):1456–1459.
- Nakata J, Suzuki Y, Suzuki H, et al. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;9(2):e89707.
- Nagayama Y, Nishiwaki H, Hasegawa T, et al. Impact of the new risk stratification in the 2011 Japanese Society of Nephrology clinical guidelines for IgA nephropathy on incidence of early clinical remission with tonsillectomy plus steroid pulse therapy. Clin Exp Nephrol. 2015;19(4):646–652.
- Kovacs T, Vas T, Kovesdy CP, et al. Effect of tonsillectomy and its timing on renal outcomes in Caucasian IgA nephropathy patients. Int Urol Nephrol. 2014;46(11):2175–2182.
- Huang H, Sun W, Liang Y, et al. CD4 (+)CD 25 (+)Treg cells and IgA nephropathy patients with tonsillectomy: A clinical and pathological study. Int Urol Nephrol. 2014;46(12):2361–2369.
- Wu G, Peng YM, Liu FY, Xu D, Liu C. The role of memory B cell in tonsil and peripheral blood in the clinical progression of IgA nephropathy. Hum Immunol. 2013;74(6):708–712.
- Suzuki Y, Tomino Y. Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal models. Semin Nephrol. 2008;28(1):66–77.