Abstract
Objectives This retrospective study determines whether the kidney disease: improving global outcomes (KDIGO) criteria are superior to acute kidney injury network (AKIN) criteria in detecting non-dialysis AKI events and predicting mortality in chronic kidney disease (CKD) patients after surgery. Methods Surgical patients who were admitted to the intensive care unit were enrolled. Non-dialysis AKI cases were defined using either KDIGO or AKIN creatinine criteria and stratified by CKD stages. The adjusted hazard ratios (AHRs) for in-hospital mortality are compared to those without AKI. The cumulative survival curves and the predictability for mortality are accessed by Kaplan–Meier method and calculating the area under the curve (AUC) for the receiver operating characteristic (ROC) curve, respectively. Results From a total of 826 postoperative patients, the overall in-hospital mortality rate was 11.6% (96 cases) and that for AKI according to KDIGO and AKIN criteria was 30.0% (248 cases) and 31.0% (256 cases). The cumulative survival curve stratified by CKD and AKI stages were comparable between KDIGO and AKIN criteria. The discriminative power for mortality stratified by CKD stages for KDIGO and AKIN criteria are as followed: all subjects: 0.678 versus 0.670 (both ps <0.001); non-CKD: 0.800 versus 0.809 (both ps <0.001); early-stage CKD: 0.676 versus 0.676 (both ps <0.001); late-stage CKD: 0.674 versus 0.660 (ps were <0.001 and 0.003). Conclusion The KDIGO criteria are superior to AKIN criteria in predicting mortality after surgery, especially in those with advanced CKD.
Introduction
Approximately 18% of patients undergoing cardiac surgery experience acute kidney injury (AKI).Citation1,Citation2 AKI increases the burden of health insurance, the length of hospitalization and in-hospital, and long-term mortality.Citation3,Citation4 The reported in-hospital mortality rate of patients with AKI ranged from 13% to 60%.Citation5–7 Thus, the diagnosis of AKI and its classification is important. Up to now, three classification systems were developed for diagnosing AKI.
The first system developed by the Acute Dialysis Quality Initiative group was Risk, Injury, Failure, Loss to End stage kidney disease (RIFLE) classification.Citation6,Citation8,Citation9 RIFLE criteria is graded by increasing the level of severity from risk, injury, failure, loss to end-stage kidney disease. The last two stages, loss and end-stage of kidney disease, were defined as clinical outcomes that correspond to a worse prognosis.Citation9 As a predictor for mortality, there are several limitations for RIFLE classifications. First, it depended directly on obtaining baseline serum creatinine (SCr) for each patient, which did not necessarily reflect the state of renal involvement. Second, there was also no definition of which specific stage indicated patients requiring renal replacement therapy (RRT).Citation9 Moreover, estimated glomerular filtration rate (eGFR) criteria were included in RIFLE classification. The use of eGFR criteria calculated as >25%, >50%, and >75% decrease for risk, injury, and failure stages has been questioned. GFR was estimated by SCr-based formulas, which is valid only when SCr is in equilibrium and steady-state.Citation5
Compared to RIFLE criteria, Acute Kidney Injury Network (AKIN) classification was proposed to take into account minor SCr changes (>0.3 mg/dL) and the need for RRT.Citation10 Besides, eGFR criteria were removed. AKIN criteria are the most restrictive of all AKI definitions, requiring a 48-h window for an acute SCr change to define AKI.Citation11 Patients started RRT and those with SCr ≥ 4.0 mg/dL with an acute increase ≥ 0.5 mg/dL was classified as stage 3 in AKIN criteria. Kidney disease: improving global outcomes (KDIGO) proposed a new set of guidelines and a definition of AKI, based on two previous classifications.Citation12 The notable difference from AKIN criteria are as followed: (1) time frame differences for absolute versus relative changes in SCr; (2) increase of ≥ 0.5 mg/dL in those with SCr ≥ 4.0 mg/dL was no longer required if minimum AKI threshold met; (3) inclusion of eGFR criteria for children.Citation13 Several studies demonstrated comparisons among RIFLE, KDIGO, and AKIN criteria.Citation5,Citation6,Citation9,Citation11,Citation14 In predicting mortality, some study showed the AKI classification systems were comparable,Citation9,Citation13 whereas some experts preferred KDIGO criteria.Citation5,Citation6,Citation11 In diagnosing AKI, it is also demonstrated the KDIGO criteria are more sensitive than AKIN and RIFLE criteria.Citation5,Citation11 However, most of these studies compared AKI staging systems (RIFLE, AKIN, and KDIGO criteria) with a logistic regression model, irrespective of the relation between time and survival.Citation5,Citation9,Citation11 Moreover, percentage rise in SCr is inadequate to diagnose AKI in those with chronic kidney disease (ACKD).Citation14 The aim of the present study was to compare the ability to identify AKI (including ACKD) and to predict mortality between AKIN and KDIGO criteria in those after surgery, after adjustment for several confounding factors of postoperative AKI, such as advanced age, emergent operation, liver disease, a high-risk operation, and chronic airway obstructive disease.Citation15 Besides, the discriminatory power for mortality of postoperative AKI in different CKD stages was also evaluated.
Materials and methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki (2000) by the World Medical Association. The protocol was approved by the Institutional Review Board of Taoyuan General Hospital (TYGH104018) and waived the need for informed consent because there was no breach of privacy or interference with decision-making processes related to patient care. This study was investigated in a single-center, and all patients in the study were directly diagnosed and followed up at Taoyuan General Hospital.
Materials
This is a retrospective observational study of 1167 patients who underwent surgery and were admitted to the intensive care unit (ICU) Department of Taoyuan General Hospital, from January of 2006 to December of 2013. Those undergoing chronic dialysis or RRT before surgery (n = 99), those less than 20 years of age (n = 7), lack of baseline demographic data (n = 20), and those who were hospitalized for less than 48 h (n = 62) were excluded. Finally, those who underwent any radiology study using intravenous contrast within the first seven postoperative days (n = 91) and those in whom AKI developed later than 7 d after surgery were excluded (n = 62) (). We investigated the relation between time and survival. Those undergoing dialysis were excluded because they might have prolonged short-term survival. RRT could confound AKI duration and survival. We also excluded those diagnosed as contrast-induced acute kidney injury (CIAKI), because the pathogenesis and prognosis are different from AKI due to other etiologies. Besides, CIAKI might be developed immediately before surgery, which is also different from postoperative AKI by definition. Consequently, a total of 826 eligible patients were included for analysis.
Methods
Demographic data, underlying disease, the severity of sepsis, surgery categories, serial renal function, the length of stay in the ICU, the length of hospitalization, the use of diuretics and vasoactive drugs, urine output, biochemical data and clinical characteristics, and ICU organ scoring system, such as Acute Physiology and Chronic Health Evaluation II (APACHE II) score, were recorded. The use of vasoactive drugs was recorded as inotrope infusion with inotrope equivalent score.Citation16 All of the included critically ill non-dialysis patients were followed until death or discharge. As previous study, the endpoint of the study is in-hospital mortality censored at 180 d.Citation17 The definitions of AKI are based on the KDIGO and AKIN criteria.Citation13 The baseline SCr, were defined as the last SCr obtained before admission, and the SCr on ICU admission and within 48 h, the initiation date for AKI and peak AKI were also recorded for KDIGO and AKIN classification. SCr was measured by Beckman Coulter AU640 analyzer. Patients with non-dialysis AKI were stratified according to the CKD stages based on proteinuria, urinalysis, SCr, and eGFR (Modification of Diet in Renal disease (MDRD4 formula)) within 3 months before admission.Citation18 The definitions of severe sepsis and septic shock are based on the 2012 International Guidelines for the Management of Severe Sepsis and Septic Shock.Citation19 The need for a ventilator was defined as those with hypoxic and/or hypercapnic respiratory failure who needed ventilator after surgery. A surgery was defined as emergent based on time criterion (surgery less than 12 h), on a place criterion (patients that come from emergent department) and on code criterion (anesthesiologist indicates an emergent code as part of the American Society of Anesthesiology physical status code).Citation15,Citation20
Statistical analysis
Continuous variables with a normal distribution are summarized as mean ± SD unless otherwise stated. Variables with a non-normal distribution are expressed as a median, the interquartile range. Based on KDIGO and AKIN criteria, patients without AKI were used as a reference group. Student’s t test, χCitation2, Mann–Whitney U, or Kruskal–Wallis tests are used to determine the differences in the demographic data, underlying disease, surgery category, scoring system, the laboratory variables, and the use of vasoactive and diuretics between patients with postoperative non-dialysis AKI and the non-AKI group. The in-hospital cumulative survival function is estimated using the Kaplan–Meier method. Cumulative survival curves for postoperative patients with different KDIGO and AKIN criteria use the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) for in-hospital mortality are calculated using the Cox proportional hazard model, with adjustment for age, gender, the need for a ventilator, the organ scoring systems, the surgery category, underlying diseases, emergent operation, the use of vasoactive drugs and diuretics, the severity of sepsis, and the baseline renal function. The discriminative ability of the criteria to predict mortality was determined using the area under the curve (AUC) for the receiver operating characteristic (ROC) curve. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL) and a p value of less than 0.05 was considered statistically significant.
Results
In total, 826 patients who underwent surgery were enrolled. None of these patients received organ transplantation. The mean age of the patients was 66.4 years. There were 608 (73.6%) males and 218 (26.4%) females. The overall incidence of in-hospital mortality was 11.6% (96 cases) and that for AKI according to KDIGO and AKIN criteria were 30.0% (248 cases) and 31.0% (256 cases), respectively. AKI cases were diagnosed according to SCr criteria (65.8% vs. 70.4%), urine output criteria (8.8% vs. 8.1%), or both (25.4% vs. 21.5%). Baseline demographical, clinical, laboratory characteristics, scoring systems, and surgical categories for non-AKI and non-dialysis AKI cases sorted by KDIGO and AKIN classification are shown in . Laboratory parameters, such as the serial renal function (blood urea nitrogen and SCr), and demographic parameters, such as the need for a ventilator, the use of diuretics, the severity of sepsis, underlying disease, and the proportions of emergent operation, are significantly differed between non-dialysis AKI and non-AKI cases. The incidence of AKI cases using KDIGO and AKIN criteria are as followed: stage 1: 9.3% versus 9.7%; stage 2: 9.7% versus 12.6%; stage 3: 11% versus 8.7% (). According to AKIN criteria, the in-hospital mortality rate was 7.0% in non-AKI and 21.8%, 20.2%, 27.8% in stage 1, stage 2, and stage 3, respectively, whereas the mortality were 6.9%, 16.9%, 17.5%, and 34.1% according to KDIGO criteria. The in-hospital mortality stratified by CKD stages and AKI classifications were showed in . Most of the patients underwent abdominal (52.4%) and cardiovascular surgery (26.1%).
Table 1. Baseline characteristics and demographic data.
Table 2. Cross tabulation of patients classified by AKIN versus KDIGO classification.
Table 3. The in-hospital mortality stratified by CKD stages and AKI classifications.
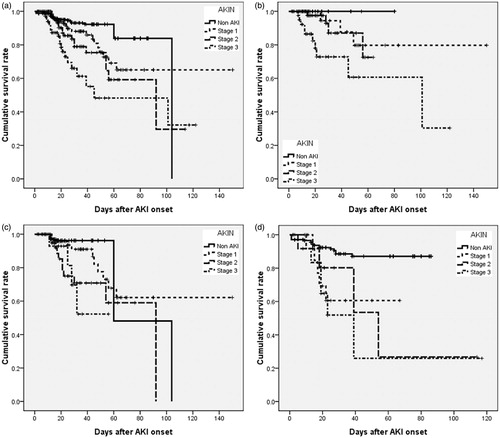
and show the in-hospital survival curve based on AKI stages and CKD stages according to KDIGO and AKIN criteria, using a log-rank test (all ps < 0.05). Compared to non-AKI group, after adjustment for confounding factors, such as age, gender, the need for a ventilator, APACHE II, surgical category, underlying diseases, emergent operation, the use of vasoactive drugs and diuretics, the severity of sepsis, the baseline renal function, the adjusted hazard ratios (AHRs) for mortality in those with non-dialysis AKI were 3.675 (2.344–5.763) (KDIGO criteria) versus 3.351 (2.130–5.271) (AKIN criteria) (both ps < 0.001).
Figure 2. The cumulative survival rate for 826 postoperative patients stratified by CKD and AKI stages using KDIGO criteria. Cumulative survival curve according to KDIGO stages for AKI in different CKD stages. (a) All subjects. (b) Non-CKD. (c) Early-stage CKD. (d) Late-stage CKD. All p values for (a–d) are <0.001.
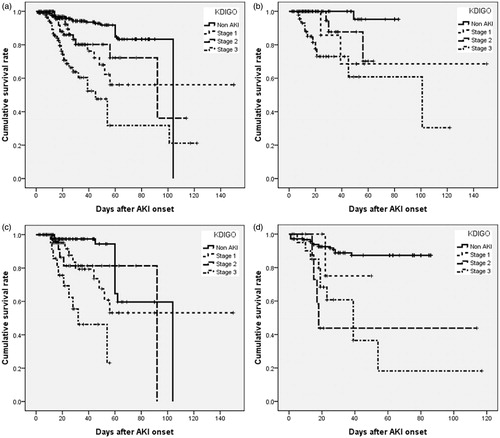
Figure 3. The cumulative survival rate for 826 postoperative patients stratified by CKD and AKI stages using AKIN criteria. The cumulative survival curves according to AKIN stages for AKI in different CKD stages. (a) All subjects. (b) Non-CKD. (c) Early-stage CKD. (d) Late-stage CKD. p Values for (a–d) are <0.001, <0.001, 0.002, and 0.001, respectively.
The multivariate-adjusted HRs (AKIN vs. KDIGO criteria) for in-hospital mortality stratified by CKD stages, compared to non-AKI group, is as follows: non-CKD group: 3.057 versus 6.671; early-stage CKD (stage 1 and 2): 5.110 versus 5.150; and late-stage CKD (stage ≥ 3): 1.844 versus 2.330 (all ps < 0.05, except both staging systems in late stage CKD). AHRs for in hospital mortality sorted by CKD and AKI stages were shown in . Since there is no significant difference for mortality between AKIN and KDIGO criteria in different CKD stages (by log-rank test), AUC for the ROC curve for mortality in different CKD stages were investigated. As shown in , the discriminative power for in-hospital mortality in KDIGO criteria is greater than that in AKIN criteria in late-stage CKD. The AUC of the ROC for different CKD stages are as followed: all subjects: 0.678 (0.615–0.740) versus 0.670 (0.609–0.730); non-CKD: 0.800 (0.703–0.897) versus 0.809 (0.728–0.889); early-stage CKD: 0.676 (0.584–0.768) versus 0.676 (0.586–0.765); late-stage CKD: 0.674 (0.563–0.785) versus 0.660 (0.549–0.770) (all ps < 0.001, except ps in those with AKI superimposed on late stage CKD, they were 0.001 vs. 0.003).
Figure 4. ROC curves and discriminatory power for death in critical ill non-dialysis patients after surgery for AKIN and KDIGO criteria, stratified by CKD stages. The AUC of ROC curves in different CKD stages. (a) All subjects. (b) Non-CKD. (c) Early-stage CKD. (d) Late-stage CKD. All p values for (a–c) are <0.001. p Value for (d) according to AKIN and KDIGO criteria were 0.003 versus 0.001.
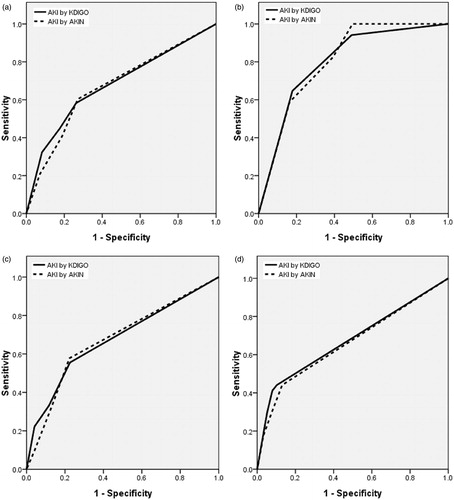
Table 4. Hazard ratios for in-hospital mortality, for different stages of AKI.
Discussion
This retrospective study demonstrates that KDIGO and AKIN criteria for AKI predict in-hospital mortality in surgical patients, after adjustment for the organ scoring systems, underlying disease, surgical category, and severity of sepsis, the baseline, and clinical characteristics. The KDIGO criteria are superior to AKIN criteria in predicting in-hospital mortality after surgery, especially in those with advanced CKD.
The validity of the AKI stage, defined by either the AKIN, RIFLE criteria, or the KDIGO classification, is a well-known predictor for mortality.Citation5,Citation6,Citation9,Citation11,Citation14 Some study showed the overall AKIN, RIFLE, and KDIGO criteria were comparable in predicting mortality,Citation9,Citation13 whereas the others showed KDIGO criteria are superior to RIFLE and AKIN criteria.Citation5,Citation6,Citation11 Some study demonstrated that KDIGO criteria detect more incident AKI than AKIN and RIFLE criteria do,Citation5,Citation11 whereas some experts do not agree with it.Citation21,Citation22 Most of these studies compared AKI staging systems irrespective of relation among time, survival, and CKD stages.Citation5,Citation9,Citation11 It is also unclear whether current staging systems to detect ACKD is appropriate or not. CKD is a well-known risk factor for AKI. Those with ACKD lead to end stage renal disease (ESRD) at a higher frequency than does AKI alone.Citation23,Citation24 Both AKI and CKD are risk factors for in-hospital mortality. The predictability of AKI stages might decrease after adjusting CKD stages. Current AKI staging systems might have lower prognostic values in CKD when based on absolute increases of SCr at the lower end of the spectrum.Citation14 Percentage rise in SCr is curtailed in CKD and is inadequate detect ACKD.Citation14 Owing to postoperative fluid accumulation with dilutional effect on SCr levels and loss of lean body mass after surgery, postoperative SCr values were lower than preoperative levels.Citation25,Citation26 For those with postoperative AKI, dilutional effect on SCr levels and loss of lean body mass might lead to minimal or no increase of SCr after surgery; thus, AKI diagnosis will be missed or delayed in a 48 h diagnostic window according to AKIN criteria, which required absolute increase of SCr ≥ 0.5 mg/dL to diagnose ACKD.Citation25,Citation26 The removal of absolute increase of ≥ 0.5 mg/dL in those with SCr ≥ 4.0 mg/dL and wide time frame difference for changes in SCr in KDIGO criteria might contribute to early diagnosis of ACKD. That is why KDIGO criteria are important and are superior to AKIN criteria in this population. We compared AKI staging systems for mortality sorted by CKD stages with Cox hazard proportional model. It demonstrated that, in predicting in-hospital mortality, KDIGO criteria are better than AKIN criteria for those with ACKD.
According to KDIGO and AKIN criteria, 8.8% (22 cases) and 8.1% (26 cases) of subjects with AKI were defined by urine output criteria. AKI might be defined more comprehensive when using data for urine output. However, they are less validated and might be influenced by several drugs, such as diuretics.Citation5,Citation27 The changed urine output caused by diuretics might result in an underestimate of the severity of the AKI when using the urine output criteria alone.Citation27 Besides, RIFLE criteria were not included in our comparisons, since it included eGFR criteria. Estimated GFR is valid only when SCr is in equilibrium and steady-state, but not for AKI.Citation5,Citation28 Oliguria and eGFR did not always show parallel changes in AKI. Moreover, patients who required RRT during admission were excluded, because RRT can confound AKI duration and survival.Citation29 The proportions of non-dialysis AKI diagnosed by KDIGO criteria is more than that of AKIN criteria in late-stage CKD (11.1% vs. 8.7%), whereas the proportions is less than that of AKIN criteria in early stage CKD group. This result might be explained by the removal of absolute increase of ≥ 0.5 mg/dL in those with SCr ≥ 4.0 mg/dL in KDIGO criteria. The minimal increase of SCr less than 0.5 mL/dL might be regarded as AKI in KDIGO criteria. AKI event is not an accurate predictor of in-hospital mortality for those with AKI less than 3 d.Citation17,Citation30 In this study, most of the AKI duration were less than 14 d (92.1%) and only 8.9% of the AKI duration were less than 48 h.Citation27,Citation29,Citation30 As the previous study, the endpoint of the present study was in-hospital mortality censored at 180 d.Citation17 However, patients might join the hospital for other reasons, especially those with prolonged hospitalization. The average length of hospitalization in similar studies was less than 30 d.Citation17,Citation25,Citation26,Citation30 In the study, most of the lengths of hospitalization are less than 30 d (78.9%). Moreover, the proportions of disagreement between staging systems are higher between KDIGO and RIFLE classifications than that of KDIGO and AKIN classifications (from 3.7% to 18%).Citation6,Citation9,Citation11,Citation14 The proportion of disagreement between different AKI staging systems is 6.8% in this study.
This study has several limitations. First, the retrospective study population is confined to one medical center and those who receiving non-cardiac surgery, so the result cannot be extrapolated to other patient populations. Second, the exclusion of patients less than 20 years of age, history of dialysis, and those suffered from AKI 7 d after surgery may lead to a selection bias. Additionally, we do not perform renal ultrasonography and renal biopsy during ICU admission routinely; therefore, obstructive AKI could not be excluded. The prognosis of obstructive AKI is different from acute tubular injury and acute glomerulonephritis. Third, the definition of baseline renal function is still controversy. Using a short time frame (SCr < 7 d before admission) or a longer time frame (SCr between 7 and 365 d before admission) for detection of AKI is different. The latter definition might detect more but less severe AKI events. RIFLE and KDIGO recommended either back calculation, longer time frame method, or the lowest SCr during hospitalization. The lack of uniform approach to estimate baseline renal function is one of the limitations. Finally, there may be residual confounding factors that could attenuate the relationship between AKI and in-hospital mortality, such as the duration of AKI and treatment strategies.Citation30
In summary, this retrospective study demonstrates that both KDIGO and AKIN criteria for AKI predict in-hospital mortality in surgical patients, after adjustment for demographic and laboratory data. The KDIGO criteria are superior to AKIN criteria in predicting in-hospital mortality after surgery, especially in those with advanced CKD. The overall incidence of AKI is similar between KDIGO and AKIN criteria. This study is limited by its retrospective nature and sample size. Multi-center, larger observational cohort studies are warranted to verify the conclusion.
Acknowledgments
Special thanks to Dr. Lin-Chien Lee in statistical analysis.
Declaration of interest
The authors report no conflicts of interest.
References
- Heringlake M, Knappe M, Vargas Hein O, et al. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72:645–654.
- Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10: 500–514.
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, lengths of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370.
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973.
- Rodrigues FB, Bruetto RG, Torres US, Otaviano AP, Zanetta DM, Burdmann EA. Incidence and mortality of acute kidney injury after myocardial infarction: A comparison between KDIGO and RIFLE criteria. PLoS One. 2013;8: e69998.
- Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818.
- Wong F, Nadim MK, Kellum JA, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60: 702–709.
- Levi TM, de Souza SP, de Magalhães JG, et al. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev Bras Ter Intensiva. 2013;25: 290–296.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, ADQI Workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Roy AK, McGorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 2013;3:26–37.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO Clinical Practice Guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
- Siew ED, Davenport A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61.
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73.
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902.
- Chen YS, Ko WJ, Lin FY, et al. Preliminary result of an algorithm to select proper ventricular assist devices for high-risk patients with extracorporeal membrane oxygenation support. J Heart Lung Transplant. 2001;20:850–857.
- Shiao CC, Ko WJ, Wu VC, et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS One. 2012;7:e42952.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Med. 2013;39:165–228.
- Catena F, Moore EE. World Journal of Emergency Surgery (WJES), World Society of Emergency Surgery (WSES) and the role of emergency surgery in the world. World J Emerg Surg. 2007;2:3.
- Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care. 2013;28: 389–396.
- Valette X, du Cheyron D. A critical appraisal of the accuracy of the RIFLE and AKIN classifications in defining “acute kidney insufficiency” in critically ill patients. J Crit Care. 2013;28:116–125.
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298.
- Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142.
- Englberger L, Suri RM, Li Z, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care Med. 2011;15:R16.
- Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: Do we have to revise current definitions of acute renal failure? Crit Care. 2008;36:1129–1137.
- Han SS, Kang KJ, Kwon SJ, et al. Additional role of urine output criterion in defining acute kidney injury. Nephrol Dial Transplant. 2012;27:161–165.
- Pickering JW, Endre ZH. GFR shot by RIFLE: Errors in staging acute kidney injury. Lancet. 2009;373:1318–1319.
- Han SS, Kim S, Ahn SY, et al. Duration of acute kidney injury and mortality in critically ill patients: A retrospective observational study. BMC Nephrol. 2013;14:133.
- Wu HC, Wang WJ, Chen YW, Chen HH. The association between the duration of postoperative acute kidney injury and in-hospital mortality in critically ill patients after non-cardiac surgery: An observational cohort study. Ren Fail. 2015;37:985–993.