Abstract
Tubulointerstitium inflammation is a common pathway aggravating chronic kidney disease (CKD) progression and the mechanism is partly associated with excessive activation of toll-like receptor 4 (TLR4) in tubulointerstitium. Ozone therapy is demonstrated to alleviate inflammation in some experiments. The aim of this study is to examine whether ozone therapy could ameliorate chronic tubulointerstitium inflammation by suppressing TLR4 in adenine-induced CKD rats. Sprague–Dawley rats were fed with 0.75% adenine-containing diet to induce CKD and tubulointerstitium inflammation injury. Ozone therapy (1.1 mg/kg) was simultaneously administrated by rectal insufflations (i.r.). After 4 weeks, serum and kidney samples were collected for detection. Renal function and systemic electrolyte were detected. Renal pathological changes were assessed by hematoxylin-eosin (H&E) staining and Masson trichrome (MT) staining. Immunohistochemistry, Western blot and Real-time PCR were applied to evaluate tubulointerstitium inflammation as well as the expression of TLR4 and phosphorylated nuclear factor kappa B P65 (p-NF-κB P65) in rats. The results showed ozone therapy improved serious renal insufficiency, systemic electrolyte disorder and tubulointerstitium morphology damages in adenine-induced CKD rats. In addition, ozone therapy suppressed excessive activation of TLR4 and p-NF-κB P65 in the tubulointerstitium of adenine-induced CKD rats, accompanied by the reduction of inflammation-related cytokines including monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6). The protein expression of TLR4 was positively correlated with the protein expression levels of MCP-1 (r = 0.7863, p < 0.01) and TNF-α (r = 0.7547, p < 0.01) in CKD rats. These findings indicated ozone therapy could attenuate tubulointerstitium inflammation injury in adenine-induced CKD rats and the mechanism might associate with the mediation of TLR4.
Introduction
Chronic kidney disease (CKD) is an irreversible disease ending in end-stage renal failure.Citation1 Irrespective of original causes, tubulointerstitial inflammation is a common, but underestimated, hallmark of CKD. This prolonged inflammation is believed to cause renal tubular degeneration and tubule-interstitial fibrosis (TIF) to aggravate the progression of CKD.Citation2,Citation3 Thus, suppressing tubulointerstitium inflammation can be an important strategy slowing the progression of CKD.
TLR4 is not only a pattern recognition receptor but also a danger recognition receptor expressed on various cell surfaces. It links natural immunity and acquired immunity and plays an important role in immune response as well as inflammation response.Citation4,Citation5 As a double-edged sword, TLR4 prevents organism from insults such as microbial infection when moderately activated, but arouses persistent inflammation cascade to impair normal tissue when excessively stimulated.Citation6 Current researchers have identified excessive activation of TLR4 contributes to the tubulointerstitial inflammation in CKD.Citation7–9 Therefore, strategies directed at inhibiting TLR4 overexpression seem to be effective to alleviate the tubulointerstitial inflammation in CKD.
Ozone is a poisonous gas in the nature, but could be applied as a safe therapeutic agent if administrated in appropriate ways.Citation10 Properties such as improving the antioxidant defense system, activating the immune system and enhancing the release of growth factors are considered as the mechanisms making ozone therapy work.Citation11 With an outstanding safety profile and high cost performance, this natural compound has applied to treat various diseases including organ ischemia reperfusion injury (IRI), herniated disks and skin ulcer in clinic.Citation12–14 Lately the anti-inflammation action of this magical treatment has been recognized by some researchers in animal experiments and the mechanisms involved in may associate with the mediation of TLR4.Citation15–18 However, whether ozone therapy could also be a promising strategy ameliorating chronic tubule-interstitial inflammation in CKD patients or CKD animal models has not been discussed yet. Therefore, this paper created a rat model of CKD and aimed at investigating whether ozone therapy could ameliorate tubulointerstitial inflammation by the regulation of TLR4.
Materials and methods
Animals
Thirty male Sprague–Dawley rats weighing 180–200 g were obtained from Tongji Experiment Animal Center, Wuhan, China. They were housed in a room with a stable temperature of 22–23 °C, humidity at 50–60% and a 12 h light–dark cycle (lights on at 6:00). They had free access to diet and water. Before any experimental intervention, the rats got 7 days to acclimatize to the new surroundings. This research was approved by the Experiment Animal Center Committee in Wuhan University and the procedure was kept in line with the international animal care guidelines.
Experiment design
According to previous researches,Citation19,Citation20 rats were fed with 0.75% adenine diet for 4 weeks to induce tubulointerstitium injury as well as irreversible CKD. Ozone therapy was performed as previously described.Citation21–23 Briefly, 3% ozone/oxygen mixture gas was generated by YKS-1000g ozone therapeutic instrument (Ikou Co., Ltd., Zhuhai, China). The ozone concentration was measured by a built-in UV spectrophotometer at 254 nm and set at 50 μg/mL. By knowing the body weight of rat, the therapeutic dose was calculated as 1.1 mg/kg which was proved to be safe and effective referring to the previous research.Citation24 Then the mixture gas was administrated by rectal insufflation with a polyethylene cannula.
All the rats were divided into three groups: Sham group (n = 6), rats were fed with normal diet and treated with nothing. ADE group (n = 12), rats were fed with 0.75% adenine diet without any treatment. OT group (n = 12), rats were fed with the same diet as ADE group did, but simultaneously administrated with ozone therapy. All the rats were sacrificed by euthanasia after 4 weeks of experimental period. Blood was obtained by the heat puncture and centrifuged at 3500 rpm for 10 min to get serum samples. The kidneys were excised and divided into two parts, one part was fixed in 4% paraformaldehyde for 24 h to make paraffin blocks at room temperature, and the rest part was frozen immediately by the liquid nitrogen. All the serum and frozen kidney samples were stored at −80 °C before detection.
Biochemical analyses
Serum creatinine (Cr), urea nitrogen (BUN) and electrolyte including potassium (K), inorganic phosphorus (IP) and total calcium (Ca) were measured by standard techniques using an Olympus AU 2700 Analyzer (Olympus Optical Co., Ltd., Tokyo, Japan).
Pathological analyses
The kidney was embedded with paraffin after 24-h paraformaldehyde fixation and sectioned to slides at 3 μm thickness. The slides were performed with hematoxylin-eosin (H&E) staining and Masson trichrome (MT) staining, then assessed under microscope. Semiquantitative analysis of MT staining was performed by using ImagePro Plus software as previously described,Citation25 10 cortical fields were analyzed at 200× magnification in each section to assess the percentage of blue collagen area relative to the whole area.
Immunohistochemical staining was performed as follows: Briefly, the slides were deparaffinized in xylene and rehydrated with ethanol gradient washes. Three percent hydrogen peroxide was applied to move endogenous hydrogen peroxide enzyme. Then the slides were retrieved from antigen by a microwave in citrate buffer liquid and blocked with 10% normal goat serum, followed by incubating with primary antibodies against TLR4 (ab8376; Abcam, Cambridge, MA), phosphorylated nuclear factor kappa B P65 (p-NF-κB P65) (Ser 536) (sc-33020; Santa Cruz Biotechnology, Santa Cruz, CA), monocyte chemoattractant protein-1 (MCP-1) (ab25124; Abcam, Cambridge, MA) and tumor necrosis factor-α (TNF-α) (ab6671; Abcam, Cambridge, MA) at 4 °C over a night. After washing three times with PBS, the slides were incubated with HRP-conjugated antibody at room temperature for 30 min. Diaminobenzidine was used to color the slides, and the staining reaction was controlled under the microscope and stopped by distilled water washes. Then the slides were counterstained with hematoxylin, finally assessed under microscope.
Western blot analyses
Total and nuclear proteins were extracted from frozen kidney and quantified by bicinchoninic acid method. Equal amounts of protein was separated on 10% SDS-PAGE, then transferred to nitrocellulose membrane and blocked with 5% nonfat milk in TBST buffer. The membranes were incubated with primary antibodies against TLR4 (ab8376; Abcam, Cambridge, MA), p-NF-κB P65 (Ser 536) (sc-33020; Santa Cruz Biotechnology, Santa Cruz, CA), MCP-1 (ab25124; Abcam, Cambridge, MA), TNF-α (ab6671; Abcam, Cambridge, MA), interleukin-1β (IL-1β) (sc-7884; Santa Cruz Biotechnology, Santa Cruz, CA) and interleukin-6 (IL-6) (sc-1266; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. After washed three times with TBST, the membranes were then incubated with the secondary antibody conjugated with horseradish peroxidase (ZSGB-BIO, Beijing, China) for 1 h at room temperature, followed by TBST washes for four times. An enhanced chemiluminescence detection kit was applied to visualize the specific bands. Quantity One software was applied to detect optical densities. The data were presented as a ratio of interest protein to GAPDH protein.
Real-time PCR analyses
Total RNA was extracted from frozen kidney using Trizol reagent (Invitrogen, Carlsbad, CA). RNA concentration was detected by spectrophotometer. RNA was obtained for reverse transcription to synthesize cDNA by using a cDNA synthesis kit (Takara, Kyoto, Japan). QPCR was applied to detect TLR4, MCP-1, TNF-α, IL-1b and IL-6 gene expression by using SYBR Green Mix kit (Applied Biosystems, Foster City, CA). GAPDH was used as a housekeeping gene and the primers are listed in . The data were presented as a ratio of genes to GAPDH mRNA.
Table 1. Primer list.
Statistics analysis
SPSS 17.0 was used for statistics analysis. Data were presented as mean ± SEM. One-way ANOVA 3 Student–Newman–Keuls test was implemented to compare the means of the different groups. Pearson correlation analysis was applied to evaluate the correlation between TLR4 and inflammation-related cytokines expression. p < 0.05 was considered as statistically significant.
Results
Effect of ozone therapy on biochemical parameters in experimental animals
Renal function was reflected by some serum parameters. Adenine diet significantly increased serum Cr, BUN, K, Ca and IP levels in rats of ADE group, while ozone therapy significantly reversed the above changes in adenine-fed rats of OT group ().
Table 2. Serum biochemical parameters in different groups at week 4.
Effect of ozone therapy on tubulointerstitium pathology
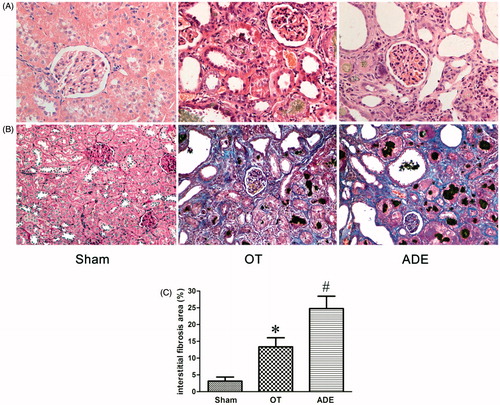
H&E staining and MT staining were performed to detect tubulointerstitium pathological changes in experimental rats. Compared with Sham group, rats in ADE group developed serious tubulointerstitium injury as evidenced by obvious tubular expansion, loss of brush border of proximal tubules, tubular atrophy and tubulointerstitium fibrosis index. No remarkable glomerular injury was observed. Changes mentioned above were mainly observed in the cortex. However, compared with ADE group, tubulointerstitium injury was significantly improved in OT group ().
Figure 1. Effect of ozone therapy on tubulointerstitium morphology at week 4. H&E staining was performed in the kidneys of different groups, original magnification ×400 (A); MT staining was performed in the kidneys of different groups, original magnification ×200 (B). Bar graphs depict the statistical analyses of relative percentages of tubulointerstitial fibrosis in the kidneys of different groups after MT staining (C). Bars represent means ± SEM. *p < 0.05 versus ADE group; #p < 0.01 versus Sham group. H&E, hematoxylin and eosin; MT, Masson trichrome.
Effect of ozone therapy on inflammatory cytokines in kidney
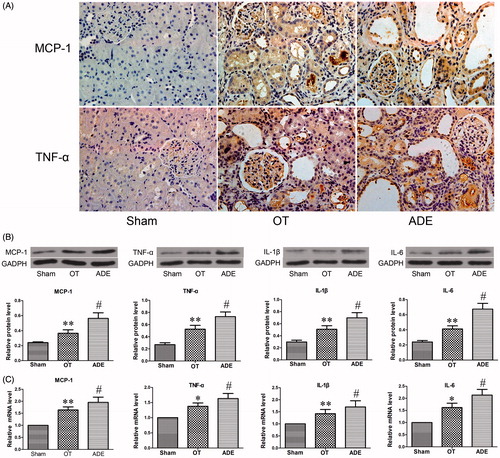
To evaluate the chronic inflammation infiltration and distribution in the kidney of experimental rats, MCP-1 and TNF-α were detected by immunohistochemical staining. In ADE group, excessive infiltration of MCP-1 and TNF-α was observed in kidney and mainly located in tubulointerstitium, while the infiltration of these cytokines in tubulointerstitium of OT group rats was significantly decreased (). To confirm the differences, more inflammatory cytokines were detected in the kidney by Western blot and Real-time PCR. Compared with ADE group, the protein and mRNA expression of MCP-1, TNF-α, IL-1b and IL-6 were significantly decreased in OT group ().
Figure 2. Effect of ozone therapy on inflammation-related cytokines in kidney at week 4. MCP-1 and TNF-α infiltration in the renal of different groups was detected by immunohistochemical staining (A), original magnification ×400. The protein level and mRNA level of MCP-1, TNF-α, IL-1β and IL-6 were respectively detected by Western blot analysis (B) and Real-time PCR analysis (C). Data were represented by mean ± SEM. *p < 0.05 versus ADE group; **p < 0.01 versus ADE group; #p < 0.01 versus Sham group.
Effect of ozone therapy on TLR4 and p-NF-κB P65 (Ser 536) in kidney
To determine whether the tubulointerstitium inflammation was related to the activation of TLR4 and NF-κB in the kidney of experimental rats, the intensity and distribution of TLR4 and p-NF-κB P65 (Ser 536) were detected. Immunohistochemical results showed TLR4 and p-NF-κB P65 (Ser 536) were both excessively expressed in tubulointerstitium, mainly in the proximal tubules, accompanied with serious inflammation-related cytokines infiltration in ADE group. However, the expression of TLR4 and p-NF-κB P65 in OT group was significantly decreased when compared with ADE group ().
Figure 3. Effect of ozone therapy on TLR4 and p-NF-KB P65 in kidney at week 4. TLR4 and p-NF-KB P65 infiltration were detected by immunohistochemical staining (A). The protein level and mRNA level of TLR4 and the protein level of p-NF-KB P65 were detected by Western blot analysis (B) and Real-time PCR analysis (C). Data were represented by mean ± SEM. **p < 0.01 versus ADE group; #p < 0.01 versus Sham group.
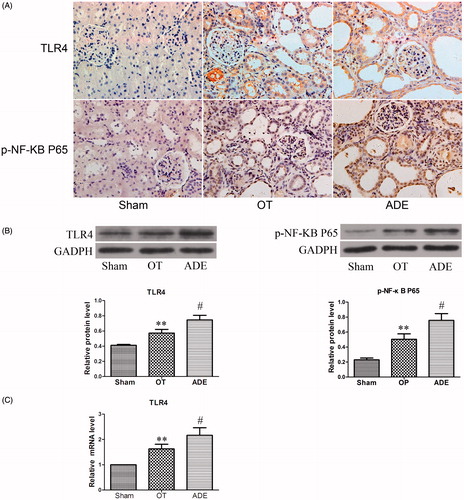
The differential expressions of TLR4 and p-NF-κB P65 (Ser 536) in different groups were further confirmed by Western blot and Real-time PCR. Compared with ADE group, the protein expression of TLR4 and p-NF-κB P65 (Ser 536) as well as the mRNA expression of TLR4 was remarkably decreased in OT group ().
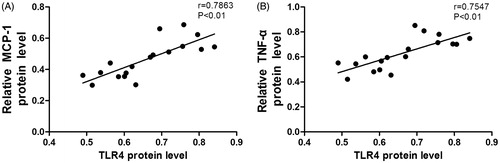
The correlation between TLR4 expression and inflammatory cytokines in kidney
As shown in , the expression of TLR4 protein was positively correlated with the protein expression levels of inflammation-related cytokines including MCP-1 and TNF-α in rats of OT group and ADE group.
Discussion
Adenine-induced CKD rodent model was firstly created by Yokozawa.Citation19 Excess adenine intake can lead to the accumulation of 2,8-dihydroxyadenine hardly soluble in urine in renal tubules, which in turn induces typical tubulointerstitium nephritis without noteworthy glomerulus injury in CKD rats.Citation20,Citation26 Compared with many other animal models of CKD, it is a stable and reliable model to study tubule-interstitial injury resembling to CKD patients.Citation20,Citation27,Citation28 The present study fed rats with adenine diet and firstly investigated the protective effect of ozone therapy to tubulointerstitium injury in adenine-induced CKD rats. When the experiment was terminated at week 4, the rats fed with adenine diet showed serious renal insufficiency, systemic electrolyte disorder and tubulointerstitium morphology damage as expected, and ozone therapy at a dose of 1.1 mg/kg by i.r. significantly improved the above-mentioned parameters in adenine-fed rats. These results firstly revealed ozone therapy could attenuate chronic renal insufficiency and tubulointerstitial injury in adenine-induced CKD rats.
Local inflammation plays an important role in tubulointerstitial injury in adenine-fed rats. Without any appropriate intervention, it would gradually lead to the conversion of tubulointerstitial inflammation to TIF and worsen renal insufficiency.Citation3,Citation29 The anti-inflammation property of ozone therapy has been made aware by some researchers. For instance, Alvarez realized ozone therapy could suppress inflammation state of rats suffering from LPS-induced endotoxic shock. Vaillant showed ozone therapy could exert anti-inflammation action to peptidoglycan-polysaccharide-induced arthritis in rats. Chen revealed ozone therapy could ameliorate acute kidney inflammation in renal IRI rats.Citation15,Citation16,Citation18 In the present paper, we firstly showed ozone therapy could decrease the infiltration of inflammation-related cytokines including MCP-1 and TNF-α in the tubulointerstitium of adenine-fed rats judging by immunohistochemistry. MCP-1, also known as chemokine (C-C motif) ligand 2, is a primary chemokine attracting monocytes and macrophages which could release proinflammation cytokines to participate in chronic inflammation cascade reaction in the process of CKD.Citation30 TNF-α is a key proinflammation cytokine that could cooperate with cytokines of interleukin family to build a pertinacious inflammation network to impair tubulointerstitium in this pathophysiological process.Citation31 Based on these data, we considered ozone therapy could suppress the tubulointerstitium inflammation in adenine-induced CKD rats and this conclusion could be further confirmed by Western blot and Real-time PCR results which showed the reductive protein and mRNA expression of MCP-1, TNF-α, IL-1b and IL-6 in the kidney of adenine-induced CKD rats after ozone therapy.
Then this paper further detected if the anti-inflammation property of ozone therapy was related to the mediation of TLR4 in the tubulointerstitium of rats fed with adenine. It is well known that during chronic tubulointerstitium injury many endogenous ligands such as heat shock protein could be continuously generated by immune cells or injured tubular cells.Citation8 As a key component of immune system, TLR4 expression in the tubule-interstitium could be excessively activated by these ligands and in turn phosphorylate and activate NF-κB via a myeloid differentiation factor 88 (Myd88) pathway. Upon activation, phosphorylated NF-κB could translocate into the nuclear and bind to special inflammatory gene sites to start transcription, followed by the production of large amounts of proinflammatory cytokines and chemokines which could damage normal tissue.Citation7,Citation32,Citation33 The activation of TLR4 signaling pathway had been revealed to contribute to the tubulointerstitium inflammation in adenine-induced CKD rats.Citation34 In consistent with the previous study, this research also observed excessive activation of TLR4 and p-NF-κB P65 (Ser 536) in the tubulointerstitium of adenine-induced CKD rats. Previously, ozone therapy was speculated to regulate TLR4 and p-NF-κB P65 (Ser 536) to suppress acute inflammation by Xing in renal IRI of rat. In this research, ozone therapy firstly exhibited its ability to suppress the excessive activation of TLR4 and p-NF-κB P65 (Ser 536) in the tubulointerstitium of adenine-induced CKD rats, which corresponds to the inflammation amelioration and the morphological improvement in this area. In addition, positive correlations between the protein expression level of TLR4 and the key inflammatory cytokines, MCP-1 and TNF-α, were observed in the present study. Taken together, we considered ozone therapy could probably alleviate the tubulointerstitial inflammation injury by suppressing immoderate activation of TLR4 in adenine-induced CKD rats.
For many years, chronic tubulointerstitium inflammation is generally recognized as a common pathway aggravating the renal insufficiency of CKD patient.Citation2 As for related mechanisms, a study on kidney biopsies of CKD patients in UK has showed upregulated renal TLR4 was closely associated with chronic tubulointerstitium inflammation in CKD.Citation9 More researches in recent years have revealed increased expression of TLR4 in tubule contributes to the tubulointerstitial inflammation in CKD.Citation8,Citation35 Therefore, strategies suppressing TLR4 could attenuate inflammation injury and slow the progression of CKD. In this paper, we firstly showed that ozone therapy could exert renoprotection effect by attenuating the chronic tubulointerstitium inflammation in adenine-induced CKD rat, and the mechanism might be partly associated with the down regulation of excessive activation of TLR4 in tubulointerstitium. Based on these data, we boldly speculated that ozone therapy, a safe and cost-effective treatment in many diseases, might also be a promising medical strategy slowing disease progression of CKD patients by decreasing tubulointerstitial inflammation. However, prior to this speculation, it was also necessary to highlight some limitations of this research: Firstly, due to the uniqueness of modeling method, our experimental model could not be applicable for all situations of tubulointerstitium inflammation in CKD. Secondly, its well-known age is an important factor influencing efficacy of therapeutic agents. Although ozone therapy is effective in improving health defense system in healthy aged rat, whether it could exert the same effect in aged CKD rats remains unknown.Citation23 Regrettably, this issue had not been investigated in this study. Thirdly, different therapeutic concentration and detection time points had not been taken into account when we evaluated the effect of ozone therapy in this research. Thus, more experimental and clinical studies are needed to confirm these results and certify our speculation.
Conclusion
Ozone therapy can alleviate the tubule-interstitial inflammation to achieve its renoprotection in adenine-induced CKD rats and the mechanism is probably related to the down regulation of TLR4.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding information
This study was supported by the Province Natural Science Foundation of Hubei (Grant No. 2013CFB226).
References
- Zandi-Nejad K, Brenner BM. Strategies to retard the progression of chronic kidney disease. Med Clin North Am. 2005;89:489–509.
- Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol. 2012;27:901–909.
- Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–1197.
- Li S, Gao X, Wu X, et al. Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim Biophys Sin (Shanghai). 2015;47:368–375.
- Robson MG. Toll-like receptors and renal disease. Nephron Exp Nephrol. 2009;113:e1–e7.
- Murad S. Toll-like receptor 4 in inflammation and angiogenesis: A double-edged sword. Front Immunol. 2014;5:313.
- Kazimierczak K, Kopec W, Klinger M. Toll-like receptors (TLR) in the pathogenesis of kidney diseases. Pol Merkur Lekarski. 2007;23:382–385.
- Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15:854–867.
- Lepenies J, Eardley KS, Kienitz T, et al. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-β1 in patients with chronic kidney disease. Nephron Clin Pract. 2011;119:c97–c104.
- Bocci V, Zanardi I, Borrelli E, Travagli V. Reliable and effective oxygen-ozone therapy at a crossroads with ozonated saline infusion and ozone rectal insufflation. J Pharm Pharmacol. 2012;64:482–489.
- Sagai M, Bocci V. Mechanisms of action involved in ozone therapy: Is healing induced via a mild oxidative stress? Med Gas Res. 2011;1:29.
- Di Filippo C, Marfella R, Capodanno P, et al. Acute oxygen-ozone administration to rats protects the heart from ischemia reperfusion infarct. Inflamm Res. 2008;57:445–449.
- Zhang J, Guan M, Xie C, Luo X, Zhang Q, Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475.
- Paoloni M, Di Sante L, Cacchio A, et al. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: A multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine (Phila Pa 1976). 2009;34:1337–1344.
- Alvarez RG, Zamora ZB, Borrego A, Delgado R, Schulz S, Alonso Y. Ozone oxidative preconditioning reduces nitrite levels in blood serum in LPS: Induced endotoxic shock in mice. Inflamm Res. 2009;58:441–443.
- Vaillant JD, Fraga A, Diaz MT, et al. Ozone oxidative postconditioning ameliorates joint damage and decreases pro-inflammatory cytokine levels and oxidative stress in PG/PS-induced arthritis in rats. Eur J Pharmacol. 2013;714:318–324.
- Xing B, Chen H, Wang L, Weng X, Chen Z, Li X. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury via modulation of the TLR4-NF-κB pathway. Acta Cir Bras. 2015;30:60–66.
- Chen H, Xing B, Liu X, et al. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol. 2008;581:306–314.
- Yokozawa T, Oura H, Okada T. Metabolic effects of dietary purine in rats. J Nutr Sci Vitaminol (Tokyo). 1982;28:519–526.
- Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant. 2013;28:2038–2045.
- Leon Fernandez OS, Ajamieh HH, Berlanga J, et al. Ozone oxidative preconditioning is mediated by A1 adenosine receptors in a rat model of liver ischemia/reperfusion. Transpl Int. 2008;21:39–48.
- Shehata NI, Abd-Elgawad HM, Mawsouf MN, Shaheen AA. The potential role of ozone in ameliorating the age-related biochemical changes in male rat cerebral cortex. Biogerontology. 2012;13:565–581.
- El-Sawalhi MM, Darwish HA, Mausouf MN, Shaheen AA. Modulation of age-related changes in oxidative stress markers and energy status in the rat heart and hippocampus: A significant role for ozone therapy. Cell Biochem Funct. 2013;31:518–525.
- Borrego A, Zamora ZB, Gonzalez R, et al. Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators Inflamm. 2004;13:13–19.
- Wang FM, Yang YJ, Ma LL, Tian XJ, He YQ. Berberine ameliorates renal interstitial fibrosis induced by unilateral ureteral obstruction in rats. Nephrology (Carlton). 2014;19:542–551.
- Zhao YY, Feng YL, Bai X, Tan XJ, Lin RC, Mei Q. Ultra performance liquid chromatography-based metabonomic study of therapeutic effect of the surface layer of Poria cocos on adenine-induced chronic kidney disease provides new insight into anti-fibrosis mechanism. PLoS One. 2013;8:e59617.
- Kadowaki D, Sumikawa S, Arimizu K, et al. Effect of acetaminophen on the progression of renal damage in adenine induced renal failure model rats. Life Sci. 2012;91:1304–1308.
- Tanaka T, Doi K, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein can reflect renal tubulointerstitial injury. Am J Pathol. 2009;174:1203–1211.
- Wang B, Ding W, Zhang M, Li H, Gu Y. Rapamycin attenuates aldosterone-induced tubulointerstitial inflammation and fibrosis. Cell Physiol Biochem. 2015;35:116–125.
- Panganiban RP, Vonakis BM, Ishmael FT, Stellato C. Coordinated post-transcriptional regulation of the chemokine system: Messages from CCL2. J Interferon Cytokine Res. 2014;34:255–266.
- Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia-the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233.
- Plociennikowska A, Hromada-Judycka A, Borzecka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557–581.
- Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231.
- Correa-Costa M, Braga TT, Semedo P, et al. Pivotal role of toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One. 2011;6:e29004.
- Yiu WH, Lin M, Tang SC. Toll-like receptor activation: from renal inflammation to fibrosis. Kidney Int Suppl (2011). 2014;4:20–25.