Abstract
Patients with chronic kidney disease (CKD) are at high risk of atherosclerotic events; dyslipoproteinemia and the decrease of the HDL-linked enzyme paraoxonase 1 (PON1), might have a major role. This study intends to compare the association between lipid profile and serum PON1 levels in renal failure (RF) and hemodialysis (HD) patients. Serum lipids, HDL-subclasses and PON1 concentration were evaluated in 90 patients with CKD, divided into groups: RF (n = 46) and HD (n = 44), and in 30 normal individuals (control group). The results showed that PON1 was significantly lower in HD patients than in RF and controls (p < 0.001). In RF patients under statin therapy, PON1 did not differ from that of patients without statins. In HD patients without statins, PON1 was considerably low, whereas in HD with statins (30.42 ± 12.62 μg/mL) was lower than RF with statins (49.31 ± 14.94, p < 0.001). PON1 concentration was significantly and positively associated with HDL-C, HDL3-C and Apo A1 in all groups. Additionally, in HD patients PON1 was negatively associated with LDL-C. Multiple regression analysis revealed that LDL-C and statin treatment were independently related to PON1 concentration in HD patients (β = −0.331, p = 0.026 and β = 0.344, p = 0.020, respectively). In RF patients, HDL3-C and Apo A1 are strong determinants of PON1 levels. It is concluded that different parameters of lipid profile seem to affect serum PON1 concentration of RF and HD patients and probably contribute to the delay of atherosclerosis.
Introduction
Human paraoxonase 1 (PON1) is an HDL-associated esterase, linked with apolipoprotein A1 (Apo A1) and clusterin (Apo J). It hydrolyzes organophosphate compounds such as paraoxon and aromatic carboxylic esters such as phenylacetate. PON1 protects HDL-C and LDL-C from oxidation,Citation1,Citation2 inhibits oxidation of lipids in macrophages and erythrocytes, and stimulates macrophage cholesterol efflux.Citation3,Citation4. Moreover, PON1 is involved in the antiatherogenic activity of HDL-C by preventing the formation of oxidized LDL.Citation5,Citation6 Most of the PON1 activity carried by HDL-C is found in the HDL3-C small subfraction of HDL-C.Citation4,Citation7,Citation8
Patients with chronic kidney disease (CKD) on hemodialysis (HD) are exposed to several risk factors for atherosclerosis, such as oxidative stress, endothelial dysfunction and dyslipidemia.Citation9–11 Dyslipidemia is mainly associated with low serum levels of HDL-C, high levels of triglycerides and elevated levels of triglyceride-rich lipoproteins or lipoprotein-remnants. Also, reduction in antioxidant defense mechanisms, such as hepatic triglyceride lipase, cholesteryl ester transfer protein (CETP), lecithin-cholesterol acyltransferase (LCAT) and PON1, exacerbate lipid abnormalities. Emerging evidence in the literature underlines the impact of decreased serum PON1 activity on the development of atherosclerotic changes in CKD patients.Citation12–14
It has been recently demonstrated that diminished PON1 activity predicts higher risk of major adverse cardiac events in CKD patients12 and that low serum concentration of PON1 may be an independent predictor of cardiovascular mortality in HD patients.Citation15 On the other hand, it has been found that statin therapy can increase PON1 activity in combination with HDL-levels and cholesterol efflux capacity in dyslipidemic patients.Citation16,Citation17
The present study aimed to compare PON1 concentration in patients with renal failure (RF) and CKD patients on HD, without or with statin therapy. We also analyzed the association of PON1 with lipids, lipoproteins, apolipoproteins, as well as HDL2 and HDL3-C subclasses, which concur in different way to HDL-C protective effect. This analysis may provide information about the anti-atherogenic part of HDL-C, as expressed by PON1, in serum of the above-mentioned groups of patients.
Materials and methods
Study subjects
The study was carried out with a total of 120 individuals, divided into three groups: RF (n = 46), HD (n = 44) and controls (n = 30). RF group consisted of patients with CKD (age 60 ± 20 years, 26 males, and 20 females) with eGFR 39 ± 17 mL/min/1.73 m2. HD group included patients with end-stage CKD (68 ± 12 years, 24 males, and 20 females) on maintenance dialysis with low-flux dialyzers for four hours three times a week. Patients were clinically stable and did not suffer from malignant, chronic inflammatory or severe liver disease. The underlying renal disorders in RF and HD groups were respectively: chronic glomerulonephritis (n = 8, n = 6), interstitial nephritis (n = 6, n = 7), polycystic renal disease (n = 7, n = 6), obstructive nephropathy (n = 5, n = 6), diabetic nephropathy (n = 6, n = 2), hypertensive renal impairment (n = 3, n = 3), systemic lupus erythematosus (n = 1 and n = 2), cardiorenal syndrome (n = 0, n = 2), nephrosclerosis (n = 3, n = 2) and unknown origin (n = 7, n = 8). The control group consisted of 30 healthy individuals (57 ± 33 years, 14 males, and 16 females), who were recruited in the same centers and had no history of kidney, inflammatory or infectious disease. A percentage of patients and controls (35–40%) were under statin therapy (10–20 g/day atorvastatin or simvastatin or rosuvastatin). All participants gave their informed consent to participate in this study that was previously approved by the Ethics Committee of the Hospital.
Biochemical assays
Blood samples were obtained after overnight fasting. In the case of HD patients, blood was obtained from intravenous fistula, just before the second weekly dialysis session. Samples were allowed to clot for 30 min, centrifuged at 2000×g for 15 min for the separation of serum, and stored at −80 °C until analysis. Cholesterol, triglycerides, HDL-C and LDL-C were measured enzymatically, while Apo A1 and Apo B were assayed by immunoturbidimetric method, using commercial kits (Roche Diagnostics, Germany). Serum PON1 concentration was measured by enzyme-linked immunosorbent assay by a commercially available kit from Uscn Life Sciences (Houston, TX). The detection limit was 1.43 ng/mL. Intra- and inter-assay coefficients were < 10 and < 12%, respectively.
Analysis of HDL-C subclasses
HDL-C subclasses were measured by homogenous HDL-C enzymatic colorimetric assay after applying a single step precipitation method.Citation18 Briefly, ApoB-containing lipoproteins and HDL2-C were precipitated in serum samples of 0.3 mL, by using 0.06 mL of a reagent containing heparin 1071 U/mL, dextran sulfate 12 mg/mL and MnCl2 500 mmol/L. After 30 min incubation and centrifugation at 10,000 rpm, HDL3-C concentration was determined in an aliquot of the supernatant and the measured value was multiplied by 1.2. HDL2-C concentration was calculated as difference between the total HDL-C and HDL3-C. This method is precise and has excellent correlation to that using ultracentrifugation.
Statistical analysis
Data were expressed as means ± SD. To apply a suitable test to each analysis, we examined whether the data showed Gaussian distribution. Depending on data distribution, unpaired t-test with Welch correction or Mann–Whitney nonparametric U-test was used for the comparison of variables between patients’ groups and controls. One-way analysis of variance (ANOVA) was used to assess the significance of differences between groups. Significance was accepted for p < 0.05 after applying the Bonferroni correction for multiple comparisons. Potential associations between PON1 and lipid parameters were assessed by linear regression analysis. In order to determine factors with independent association with serum PON1, multiple regression analysis was performed. All analyses were carried out using SPSS statistical package, version 13.0 (SPSS Inc., Chicago, IL) for Windows.
Results
The clinical characteristics of all study participants are summarized in . There was no significant difference in age, gender and body mass index between groups. The percentage of individuals who were under statins therapy was similar in all groups.
Table 1. Demographic characteristics of patients and controls.
The lipid profile of patients and controls is presented in . Patients with RF had significantly higher triglycerides (p < 0.01) and lower HDL-C (p < 0.01) and Apo A1 levels than controls (p < 0.05), but did not differ in total cholesterol, LDL-C and Apo B. Patients on HD had major differences in all studied lipid parameters compared to controls and had significantly lower levels of total cholesterol and LDL-C than RF patients (p < 0.01 and p < 0.001, respectively). HDL-C concentration had a downward trend from controls to RF and from RF to HD patients (p < 0.01). Regarding HDL-C subclasses, the HD patients, as RF patients, had lower HDL2-C levels compared to controls (p < 0.01); moreover HD patients had considerably reduced HDL3-C compared to RF and controls (p < 0.001). PON1 concentration was decreased in RF patients compared to controls but this decrease was not statistically significant. PON1 concentration in HD patients followed HDL-C and HDL3-C concentrations and was lower than in controls (p < 0.001) and also than in RF patients (p < 0.001). As concerns the statin sub-groups, shows that either RF or HD patients treated with statins have significantly increased levels of HDL3-C compared to the same group without statins (p < 0.001). Additionally, HD patients treated with statins had lower levels of HDL-C (p < 0.05) and HDL3-C (p < 0.001) than RF patients with statins, but similar levels to RF patients without treatment. PON 1 concentration in each group treated with statins was increased compared to the same non-treated group () but this increase was not significant. PON1 levels in HD patients without statin treatment were significantly lower (p < 0.001) than RF patients with but also without statins (p < 0.001). On the contrary, in HD patients with statins, PON1 differed only from that in RF with statins (p < 0.001).
Figure 1. Comparison of serum PON1 levels in RF and HD patients with or without (w/o) statin treatment.
Note: *p < 0.001 versus RF without statins and RF with statins, **p < 0.001 versus RF with statins.
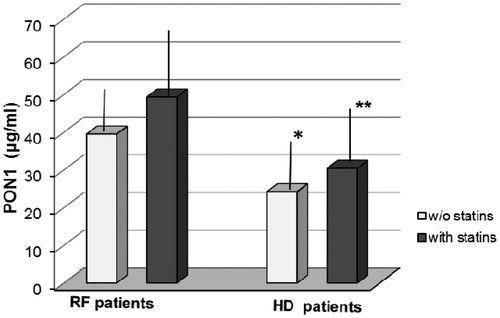
Table 2. Lipid profile and PON1 concentration in patients and controls (C).
Table 3. Lipid profile and PON1 concentration in CKD patients without (w/o) and with statins.
shows linear regression analysis between PON1 and the studied lipid parameters in CKD patients. PON1 concentration was significantly and positively associated with HDL-C, HDL3-C and Apo A1 in RF and HD groups. Additionally, there was strong negative correlation between PON1 and LDL-C only in HD patients (r = −0.423, p = 0.014). The parameters which showed significant (p < 0.05) association with PON1 (i.e., HDL-C, HDL3-C, LDL-C and Apo A1) together with statin uptake, were included in a model of multiple regression analysis (). As observed, PON1 was independently associated with HDL3-C (β = 0.332, p = 0.010) and Apo A1 (β = 0.298, p = 0.026) in RF group. However, in HD patients, LDL-C levels and statin treatment were independent factors associated with PON1 concentration (β = −0.331, p = 0.026 and β = 0.344, p = 0.020, respectively).
Table 4. Linear regression analysis between serum PON1 concentration, lipids and apolipoproteins in CKD patients.
Table 5. Multiple regression analysis of lipid-associated factors influencing serum PON1 concentration of CKD patients.
Discussion
Our results show that patients with RF have differences in the lipid profile compared to CKD patients on HD and that these differences affect serum PON1 concentration. The most important are the higher levels of triglycerides and lower levels of HDL-C in both patients’ groups compared to controls, and the considerably decreased levels of HDL and HDL3-C in HD patients compared to RF. Moreover, the atheroprotective protein Apo A1 is significantly reduced in patients compared to healthy individuals. Disturbed lipoprotein profile has also been reported in several reportsCitation14,Citation19,Citation20 and was ascribed to enhanced oxidative stress, malnutrition and inflammatory disorders, especially in patients subjected to maintenance HD. These lipid alterations are accompanied in our study by a significant decrease of PON1 concentration in HD patients compared to RF and controls, a fact that probably reflects the higher degree of lipid peroxidation in HD group. The observed PON1 levels are similar to those determined elsewhere in pre-dialysisCitation21 and dialysis patients.Citation22 However, as far as our literature survey was concerned, this study provides novel comparison data about PON1 concentration between the above CKD modalities.
The decrease in PON1 activity is known to be associated with low and/or dysfunctional HDL in pre-dialysis patients, as well as with decreased serum arylesterase and paraoxonase activities in CKD patients,Citation12–14,Citation23 leading to diminished protection against lipoprotein oxidation. Nevertheless, recent studies point to the significance of PON1 concentration and not of paraoxonase or arylesterase enzymatic activity as independent predictor of cardiovascular mortality in HDCitation15 and also as a better atherosclerotic risk factor than HDL-C in type 2 diabetes mellitus.Citation24 Statins, apart from the capacity of lowering cholesterol levels by inhibiting the enzyme HMG-CoA reductase, provide anti-oxidative protection by increasing PON1 mass and activity.Citation16 Considering that 35–40% of the patients of our study were under statin therapy, we may assume that in those patients statins have protective effect on PON1 levels, a fact that was confirmed by the increase of HDL3-C and not HDL2-C levels. As known, HDL3-C subclass constitutes a large proportion of antioxidant enzymes within HDL, including PON1, whereas HDL2-C is mostly involved in removal of cholesterol in patients with end-stage renal disease.Citation7,Citation25 The higher HDL3-C subclass levels which were shown to be related to higher PON1 activity,Citation4,Citation7 may possibly explain the marginally increased PON1 concentration observed in patients treated with statins of the present study.
Linear regression analysis verified the strong associations between PON1 HDL-C and HDL3-C concentrations in RF and HD groups. Moreover, it was found that PON1 is significantly associated with Apo A1. In general, Apo A1 is directly related to the number of HDL particles, as it is the major protein component of HDL; thus, the low levels of Apo A1 found in patients and, therefore, of HDL particles may be consistent with the low PON1 concentration. PON1 concentration was also significantly correlated with LDL-C, but only in HD group. Although LDL-C levels are low in HD patients compared to the controls, the negative correlation between PON1 and LDL-C probably suggests that higher LDL-C levels in some of the patients could lead to lower PON1 concentrations and consequently higher risk for atherosclerotic events. This may be probably supported by the observations that LDL-C and the oxidation of LDL-C phospholipids inhibit the synthesis of PON1 in the liver and play a critical role in developing atherosclerosisCitation26 and also that oxidized LDL concentration is increased in serum of HD patients.Citation11,Citation13
In previous report,Citation27 in a statistical model including genetic factors influencing PON1 activity and non-genetic factors (lipids, CRP, RHuEPO dose, etc.), it was found that Apo A1, VLDL and LDL levels were independent variables associated with PON1 in HD patients, whereas triglycerides were main determinants in HD patients who were not under statin therapy. Certainly, the above results are not comparable with those obtained from our model of multiple regression analysis, which includes different parameters. However, it seems that low Apo A1 and low HDL3-C are independent predictors for decreased PON1 levels in RF patients, while high LDL-C and no statin treatment independently predispose to decreased serum PON1 concentration in HD patients. It should be noticed that the heterogeneity of statin therapy (atorvastatin, simvastatin or rosuvastatin) and the small sample size in the sub-groups of patients (without or with statins) probably pose some limitations. Therefore, larger population studies analyzed according to lipid-lowering therapy, could give a better insight about the effect of statins on PON1 atheroprotective action.
In conclusion, our results show that the decrease of serum PON1 concentration is influenced by the lipid profile of CKD patients. HDL3-C and Apo A-1 concentrations are strong determinants of PON1 in RF patients. Low LDL-C and statin therapy are positively associated with PON1 levels in HD patients, a fact that probably contributes to the delay of atherosclerotic changes in their asymptomatic phase.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Pamo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590.
- Kaysen G. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr. 2009;19:73–77.
- Rosenblat M, Volkova N, Ward I, Aviram M. Paraoxonase 1 (PON1) inhibits monocyte-to-macrophage differentiation. Atherosclerosis. 2011;219:49–56.
- Gugliucci A, Kotani K, Kimura S. Paraoxonase 1 in chronic kidney failure. J Lipids. 2012;2012:726048.
- Mackness M, Mackness B, Durringrton PN. Paraoxonase and coronary heart disease. Atherosclerosis Suppl. 2002;3:49–55.
- Precourt LP, Amre D, Deni MC, Lavoie JC, Delvib E, Seldman E. The three-gene paraoxonase family: Physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36.
- Schiavon R, Battaglia P, De Fanti E, et al. HDL3-related decreased serum paraoxonase (PON) activity in uremic patients: Comparison with the PON1 allele polymorphism. Clin Chim Acta. 2002;324:39–44.
- Kontush A Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Ather Thromb Vasc Biol. 2003;23:1881–1888.
- Lewis D, Haynes R, Landray M. Lipids in chronic kidney disease. J Ren Care. 2010;36:27–33.
- Vaziri N, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010;6:287–296.
- Samouilidou E, Karpouza A, Grapsa E, Tzanatou-Exarchou H. Serum oxidized LDL is inversely associated with HDL2-cholesterol subclass in renal failure patients on hemodialysis. Nephron Clin Pract. 2010;115:c289–c294.
- Kennedy D, Tang W, Fan Y, Wu Y, Mann S, Pepoy BS. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2:e000104. doi:1161/JAHA.112.000104.
- Johnson-Davis K, Fernalius C, Eliason N, Wilson A, Beddhu S, Roberts W. Blood enzymes and oxidative stress in chronic kidney disease: A cross sectional study. Ann Clin Lab Sci. 2011;41:331–339.
- Kotur-Stevuljevic J, Peco-Antic A, Spasic S, et al. Hyperlipidemia, oxidative stress, and intima media thickness in children with chronic kidney disease. Pediatr Nephr. 2013;28:295–303.
- Ikeda Y, Suehiro T, Itahara T, et al. Human serum paraoxonase concentration predicts cardiovascular mortality in hemodialysis patients. Clin Nephrol. 2007;67:358–365.
- Harangi M, Seres I, Harangi J, Paragh G. Benefits and difficulties in measuring HDL subfractions and human paraoxonase-1 activity during statin treatment. Cardiovasc Drugs Ther. 2009;23:501–510.
- Miyamoto-Sasaki M, Yasuda T, Mongushi T, et al. Pitavastatin increases HDL particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J Ather Thromb. 2013;20:708–716.
- Hirano T, Nohtomi K, Koba S, Muroi A, Ito Y. A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay. J Lipid Res. 2008;49:1130–1136.
- Puchades MJ, Saez G, Munez MC, et al. Study of oxidative stress in patients with advanced renal disease and undergoing either hemodialysis or peritoneal dialysis. Clin Nephrol. 2013;80:177–186.
- Kimak E, Ksiazek A, Solski J. Disturbed lipoprotein composition in non-dialyzed, hemodialysis, continuous ambulatory peritoneal dialysis and post-transplant patients with chronic renal failure. Clin Chem Lab Med. 2006;44:64–69.
- Marsillach J, Martinez-Vea A, Mareas L, Mackness B, Mackness M, Ferré N. Administration of exogenous erythropoietin beta affects lipid peroxidation and serum paraoxonase-1 activity and concentration in predialysis patients with chronic renal disease and anaemia. Clin Exp Pharmacol Physiol. 2007;34:347–349.
- Suehiro T, Ikeda Y, Shiinoki T, et al. Serum paraoxonase (PON1) concentration in patients undergoing hemodialysis. J Atheroscler Thromb. 2002;9:133–138.
- Gugliucci A, Kinusa E, Ogata H, Caccavello A, Kimura S. Activation of paraoxonase 1 after hemodialysis is associated with HDL remodeling and its increase in the HDL2 fraction and VLDL. Clin Chim Acta. 2014;430:9–14.
- Patra SK, Singh K, Singh R. Paraoxonase 1: a better atherosclerotic risk predictor than HDL in type 2 diabetes mellitus. Diabetes Metab Syndr. 2014;7:108–111.
- Alabakova S, Todorova S, Labudovic D, Tosheska K. LDL and HDL subclass distribution in patients with end-stage renal diseases. Clin Biochem. 2002;35:211–216.
- Navab M, Berliner JA, Subbanagounder G, et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488.
- Ribeiro S, do Sameiro Faria M, Mascarenhas-Melo F, et al. Main determinants of PON1 activity in hemodialysis patients. Am J Nephrol. 2012;36:317–323.
