Abstract
Recent studies have suggested that some blood physicochemical and urinary biochemical parameters have a standardized behavior during acute kidney injury (AKI) development. The changes in these parameters frequently begin to occur before significant rises in serum creatinine (sCr) and may help in identifying patients with more subtle decreases in glomerular filtration rate (GFR). Surgical patients have an increased risk of AKI but renal impairment is usually not evident at ICU admission. We hypothesized that the surgical patients who have AKI diagnosed in the early postoperative period have an impaired GFR since ICU admission, indirectly inferred by alterations in these blood physicochemical and urinary biochemical parameters even in the presence of a still normal sCr. We retrospectively evaluated 112 surgical patients who were categorized according to AKI development during the first 3 ICU days. Twenty-eight patients developed AKI, most of them in the first day (D1) after ICU admission (D0). AKI patients had, at D0, lower serum pH and albumin, higher C - reactive protein (CRP), lower urine sodium (NaU) and fractional excretion of urea (FEUr). Fractional excretion of potassium (FEK) was high in both groups at D0 but remained high in the subsequent days only in AKI patients. Very low CRP and high serum albumin, high NaU and FEUr values at ICU admission had a significant negative predictive value for AKI. We concluded that some easily assessed parameters in blood and urine may help to identify patients with indirect signs of increased inflammatory response and decreased GFR at ICU admission, which could help to predict the risk of postoperative AKI development.
Introduction
Acute kidney injury (AKI) is a threatened complication of major surgical procedures.Citation1,Citation2 A high incidence of AKI (almost 50%) has been recently described in critically ill surgical patients.Citation2 AKI leads to increased intensive care unit (ICU) and hospital stay, increased costs, morbidity and mortality.Citation3 Hence, early AKI diagnosis remains a challenge which could help in its adequate management and improve prognosis. Many markers of tubular damage have been proposed to be of clinical utilityCitation4 but they are expensive and not widely available especially in developing countries.
We have recently described the differences in the sequential behavior of blood physicochemical parameters and urinary biochemistry of critically ill patients who had or had not developed AKI.Citation5 In that study, AKI development was characterized by increases in the strong ion gap (SIG) and serum phosphate besides decreases in urinary sodium (NaU), chloride (ClU) and increases in urinary strong ion difference (SIDu), alterations which preceded increases in serum creatinine (sCr) and decreases in urine output. In another complementary study,Citation6 we have suggested that increases in the fractional excretion of potassium (FEK) may be surrogates of decreases in glomerular filtration rate (GFR) and also precede increases in sCr. Of note, in these previous studies,Citation5,Citation6 most of the patients had a medical reason for ICU admission. Surgical patients, however, are expected to have a more precise and homogeneous moment that AKI is usually triggered, which is the surgical procedure itself. Therefore, it would be of interest to follow blood and urinary parameters since ICU admission considering that most AKI cases would be diagnosed early in the postoperative period but probably not on arrival. It is possible, however, that some degree of renal impairment is already present on arrival but not sufficiently severe to increase sCr, which has low sensitivity in detecting initial decreases in GFR.
In addition, most AKI developing in the first postoperative days are caused, at least in part, by systemic inflammatory response syndrome (SIRS), so that usual parameters to quantify inflammation (leukocytes count, serum c-reactive protein (CRP)) are also expected to behave distinctly in patients who develop or not AKI in the early postoperative period. It was previously demonstrated that CRP is a good predictor of major postoperative complications, including AKI, when measured preoperatively in cardiac surgery.Citation7 We hypothesized that sequential evaluation of blood physicochemical parameters; urinary biochemistry as well as routinely used markers of systemic inflammation would reveal distinct profiles (perhaps since ICU admission) between patients who develop or not AKI in the first days of the postoperative period.
The primary objective of the present study was to describe and characterize the behavior of these parameters in a population of surgical patients admitted in the immediate postoperative period, separating patients who had or had not developed AKI in the first three days of ICU stay. As a secondary objective, we evaluated blood and urinary parameters that could have some potential in predicting AKI development when measured early in the postoperative period.
Materials and methods
The project was approved by the Local Ethics Committee (protocol number 23180613.7.0000.0062). From February to August 2013, a spot urine sample was part of the exams collected from every patient admitted in the ICU with an indwelling urinary catheter. Urinary sodium (NaU), potassium (KU), chloride (ClU), urea (UrU) and creatinine (CrU) were measured at ICU admission and then daily simultaneously with the routine blood exams (around 4:00 AM) as long as the patient remained with the urinary catheter. The decision to remove the catheter was at the discretion of the attendant physician. We retrospectively analyzed the data available from the surgical patients. Patients with no urinary catheter, history of chronic renal failure, renal transplantation or bladder irrigation were excluded. In case of more than one ICU postoperative admission, only one surgical admission was considered for each patient. Admission and routine blood exams consisted of: complete blood cell count, c-reactive protein (CRP), serum urea (Ur) and sCr, Na+, K+, Cl-, Ca2+, Mg2+, phosphate, albumin, venous blood gas and lactate.
Anion gap (AG), apparent SID (SIDa), effective SID (SIDe), SIG and SIDu were calculated as (all in mEq/L):
(1)
(2)
(3)
(4)
(5)
Fractional excretions of Na+ (FENa), K+ (FEK) and Ur (FEUr) were calculated as:
D0 was considered the day of ICU admission (first set of blood and urinary exams at ICU arrival) and D1, the subsequent day (first routine blood and urinary exams collection). For the purpose of this study, the observation period included only data from D0 to D3 of the patients admitted in the immediate postoperative period. Patients with at least two urinary samples from D0 to D3 were included in the final analysis. Postoperative patients were followed until urinary catheter removal or until D3, whichever occurred first. AKI patients were defined as patients who developed AKI at any day during the observation period. AKI diagnosis was made according to sCr-based Acute Kidney Injury Network (AKIN) criteria.Citation8 Since most patients were admitted electively into the hospital for the purpose of the surgery, AKI at ICU admission was evaluated using the first sCr after hospital admission as baseline creatinine. Vasopressor, diuretic use, mechanical ventilation, 24 h urine output, 24 h fluid input and need of renal replacement therapy during the observation period were recorded. SAPS 3 risk of mortalityCitation9 at ICU admission and ICU and hospital mortalities were also evaluated.
Statistical analysis
The comparison of the general characteristics between no-AKI and AKI patients were made using the Rank Sum Mann-Whitney test for continuous variables or Chi-square/Fisher exact test for categorical variables, as appropriate. Daily comparisons of the parameters between the two groups were made using the Rank Sum Mann-Whitney test. Most data had a non-parametric distribution so that the values were presented as median of 25th and 75th percentiles. Correlations between variables were made using the Spearman Rank coefficient. The area under the curve and 95% confidence interval were calculated based on the receiver operating characteristic analysis. A p-value less than 0.05 was considered significant. Statistical analyses were made using the SigmaPlot software version 12.0 (Systat Software Inc, San Jose, CA).
Results
During the study period, 112 out of a total of 218 surgical admissions fulfilled the inclusion criteria and entered in the analysis. General characteristics of both no-AKI and AKI patients are presented in . Most of the surgeries were considered elective procedures in both groups. Twenty-eight patients (25%) developed AKI during the observation period. AKI patients were older, with an increased prevalence of arterial hypertension and diabetes mellitus, higher baseline sCr, more severely ill (higher SAPS3 and use of ICU support) and had an increased mortality both in the ICU (not statistically significant) and in the hospital. Chronic organ failures were uncommon in both groups. Most of the AKI diagnoses were made at D1 and the majority of the AKI patients had only a mild increase in sCr (maximum AKIN stage 1) during the observation period.
Table 1. General characteristics of the 112 surgical patients included in the analysis.
Traditional acid-base and renal parameters according to acute kidney injury development
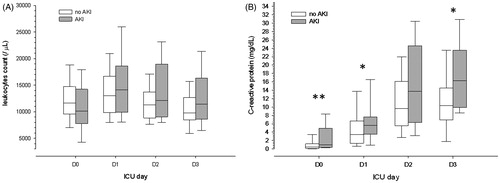
Blood pH was significantly lower at all days in AKI patients (). The lower pH was attributed to discretely lower SBE and discretely higher PCO2 in AKI patients. Both groups had a mild hyperlactatemia at D0 which resolved similarly in both groups along the days. The AG was significantly higher in AKI patients at D2 and D3. Both serum urea and sCr were significantly higher in AKI patients since ICU admission, even though only a minority of the patients had AKI diagnosed at D0 (). Median serum potassium was in the normal range at both groups at all days.
Table 2. Traditional acid-base and renal parameters according to acute kidney injury status in the early postoperative period.
Traditional markers of systemic inflammation according to acute kidney injury development
Leukocytes count was similar between groups at all days (). Mild leukocytosis was observed in both groups at D1, decreasing similarly in both groups in the subsequent days. Median CRP level was very low in both groups at ICU admission but already significantly higher in AKI patients. CRP value increased until D3 in both groups but more pronounced in AKI patients ().
Blood physicochemical parameters according to acute kidney injury development
Apparent SID value was very similar between groups at all days but lower SIDe values were observed in AKI patients at D1 and D2 (). SIG value was similar at ICU admission and increased in both groups in the subsequent days but significantly more pronounced in AKI patients (). Serum albumin was significantly lower at ICU admission in AKI patients. There was a progressive decrease in serum albumin along the days but only in no-AKI patients, so that serum albumin levels were very similar between groups at D2 and D3. Median serum phosphate was in the normal range at both groups at all days. However, serum phosphate decreases along the days in no-AKI patients, being significantly lower than in AKI patients at D2 and D3.
Figure 2. Differences in strong ion gap evolution in the first three postoperative days between patients who developed or not AKI during this period. Notes: ICU: intensive care unit. AKI: acute kidney injury. *p < 0.05; **p < 0.01; ***p < 0.001.
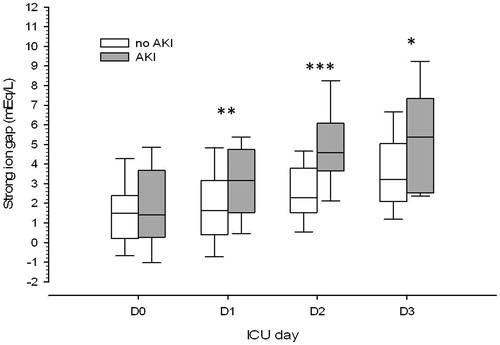
Table 3. Blood physicochemical parameters according to acute kidney injury status in the early postoperative period.
Urinary biochemical parameters and indices according to acute kidney injury development
NaU and ClU values were lower at all days in AKI patients (). KU value was similar between groups at all days, except D3. UrU value was higher in no-AKI patients at D1 and D2 but CrU value was similar between groups at all days. SIDu value was similar between groups until D3, in which higher values were found in AKI patients. FENa values were similar between groups at all days. FEUr values were statistically lower in AKI patients only at ICU admission. FEK values were significantly higher in AKI patients from D1 to D3. It decreased progressively in no-AKI patients, reaching normal values at D2-D3.
Table 4. Urinary biochemical parameters and indices according to acute kidney injury status in the early postoperative period.
Correlation between serum creatinine and fractional excretions of sodium, potassium and urea
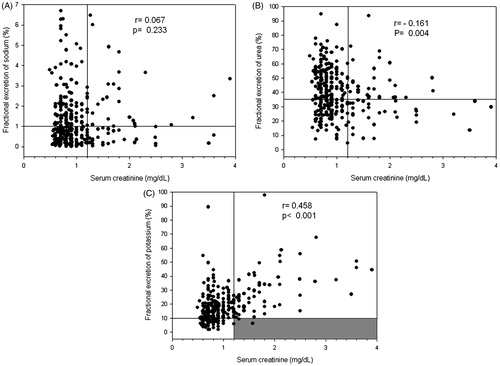
In the early postoperative period, there was no correlation between sCr and FENa (). Considering normal sCr values as lower than 1.2 mg/dL and 1% as a classical cutoff value for FENa,Citation10 there seems to have a homogeneous distribution of FENa above or below 1% for both normal and increased sCr values. A similar distribution pattern was found for FEUr considering a cutoff value of 35%Citation11(). This explains the poor correlation between sCr and FEUr albeit statistically significant. The correlation between sCr and FEK was found to have a distinct pattern and to be more clinically relevant than the other two fractional excretions. Notably, patients with increased sCr almost always had an increased FEK (above 10%Citation12) ().
Figure 3. Correlation between serum creatinine and fractional excretion of sodium (A), urea (B) and potassium (C). Horizontal lines represent the usual cutoffs for the fractional excretions (1%, 35% and 10%, respectively); vertical lines represent the cutoff for normal serum creatinine levels (considered less than 1.2 mg/dL). The gray zone highlights the almost absence of samples with increased serum creatinine and simultaneous normal fractional excretion of potassium.
Diagnostic performance of different parameters measured at ICU admission in predicting acute kidney injury in the early postoperative period
Some blood and urinary parameters were already significantly different between groups at ICU admission (see above). In order to evaluate the accuracy of these parameters at ICU admission in predicting AKI in the early postoperative period, we performed receiver-operating characteristic curve analysis. CRP, serum albumin, NaU and FEUr had a fair but significant accuracy at ICU admission in predicting AKI development (). Since FEK was found to have distinct behaviors from D0 to D1 according to AKI development (see above), we have also evaluated the diagnostic performance of Δ FEK (FEK at D1 to FEK at D0) in predicting AKI development in the early postoperative period. We have found a good accuracy and high specificity and predictive values (). We have also evaluated ΔSIG and ΔCRP in the same way as ΔFEK but they did not have significant accuracies in predicting AKI development.
Table 5. Diagnostic accuracy, sensitivity, specificity, positive and negative predictive values for AKI diagnosis in the early postoperative period of distinct urinary and blood parameters measured at ICU admission.
Discussion
In this retrospective analysis, we compared blood and urinary parameters among surgical patients who developed or not AKI in the early postoperative period. We have previously suggested that some of these parameters may be useful in the early AKI diagnosis.Citation5,Citation13,Citation14 However, in these studies, most AKI diagnoses were made at ICU admission, limiting the data available at the days preceding AKI. In the present study, we have found that a minority of the patients had AKI diagnosed at ICU admission (D0), which is not surprising in a population composed mainly by elective surgical patients. Interestingly, we still have found a high incidence of AKI (25%) manifesting early in the postoperative period, usually at D1 and D2 () confirming that AKI was related to the surgical procedure itself and that it is usually an early postoperative complication. Such a high AKI incidence could be due to the fact that the patients included in the analysis were those who were considered of high risk of complications, remaining with their urinary catheter and in the ICU for longer periods.
Although most AKI diagnoses were made at D1, some parameters were already different between groups at D0. AKI patients had, since ICU admission, more significant acidemia and higher serum urea and sCr, findings that suggest some degree of renal impairment already present at D0 in most AKI patients even though a minority of the AKI patients had serum urea and sCr increased enough at D0 to reach an AKIN stage of AKI. It is noteworthy that median baseline sCr had normal values in both groups but higher value in AKI patients suggesting that these patients had a lower creatinine clearance and GFR (a lower renal function “reserve”) even before surgery. Therefore, we believe that, even in the normal sCr range, a higher baseline sCr or a higher sCr at ICU admission may indicate an increased risk of early postoperative AKI development. This “risk” may actually represent a subclinical decrease in GFR. It is possible that GFR begins to decrease during or soon after the surgery but, if it is not so abrupt, sCr at admission is not able to reveal such decrease.
We have recently demonstrated the relevance of measuring sCr more frequently and concomitant with urine biochemistry in the early postoperative period after cardiac surgeries.Citation15 In patients who developed AKI, increases in sCr and parallel abrupt decreases in NaU occurred in hours after ICU admission, probably as a result of significant decreases in GFR. In the present study, NaU values were also lower at ICU admission in AKI patients (), possibly also representing an early decrease in GFR. Although not significantly different at admission, higher FEK values in AKI patients during the entire observation period () may also represent GFR impairment. Distinct from FENa, which seems to be of no value in AKI monitoring,Citation16 FEK was recently described as a valuable monitoring tool.Citation6 This finding was confirmed by the fact that low FEK values (< 10%Citation12) were only present in patients with low sCr (), being a specific marker of normal renal function similar to very high NaU values, as previously demonstrated.Citation17 Low FEK and high NaU may actually represent a less activated sympathetic and renin-angiotensin-aldosterone systems, both implicated in AKI pathophysiology.Citation18 Decreases in FEK along the days in no-AKI surgical patients may represent a non-sustained activation of these systems and better renal outcome. A higher NaU could also be a surrogate of a more aggressive sodium-rich fluid resuscitation but, for the patients that we have complete data regarding fluid balance, there was no difference in fluid input between patients who developed or not AKI in the first three ICU days after surgery (). Unfortunately, we don’t have the data regarding intra-operative fluid management and the types of fluids used. Although fluctuations in urinary values are expected in a 24-h period, we believe, based on recent publications,Citation5,Citation13,Citation19 that a single spot urine biochemistry assessment per day, usually at the same hour (together with the routine blood exams) is useful and quite more practical than in a 24-h urine sample.
The utility of FEUr measurement in AKI is still a matter of controversy but, in our study, FEUr was significantly lower in AKI patients at ICU admission (), which might suggest a greater avidity for urea reabsorption in this group, possibly a result of lower GFR. This finding is corroborated by higher serum urea since D0 () and lower UrU levels in AKI patients at D1 and D2 ().
Our results demonstrated a standardized behavior of some parameters after surgery independently of AKI development. Transient increases in leukocytes count and lactate as well as progressive increases in both SIG and CRP in the first three postoperative days are some of the changes triggered by major surgeries, as previously described.Citation20 We could speculate that the intensity of such changes is related to the degree of the inflammatory response and AKI development. In this issue, lower serum albumin (which is a negative acute phase protein) and higher CRP at ICU admission in AKI patients might be a result of a more intense inflammatory process. Only a minority of the patients had an infectious cause of the surgery (7.1% in no-AKI vs. 10.7% in AKI patients, p = 0.841), so that we do not believe that the differences in CRP levels at admission could be attributed to a different prevalence of SIRS/sepsis previously to surgery. Greater increases in SIG in AKI patients along the days are indeed difficult to interpret because it may be both a result of the delivery of a higher concentration of unidentified anions into the circulation during SIRS but also may be due to their retention as a result of renal impairment itself.Citation21
Other blood and urinary physicochemical parameters did not seem to be of major relevance in postoperative AKI monitoring. SIDa and SIDe were not found to have different values at ICU admission between groups and both had stable values along the observation period. SIDu was significantly different only lately (at D3) due to progressive increases in AKI patients. The same applies to the AG and phosphorus, which were only different between groups lately.
Considering that postoperative AKI is prevalent and associated with poor prognosis and that AKI diagnosis is usually based on sCr which is a late marker of GFR impairment, our article is the first to suggest that some easily assessed (even in low resource ICUs) blood and urinary parameters are potentially useful in AKI monitoring. The negative predictive values of the cutoff points of CRP, serum albumin, NaU and FEUr at ICU admission () are of particular interest, since they may be markers of lower risk of developing postoperative AKI. In other words, these parameters may be related to the magnitude of the surgical inflammatory trigger, which is probably the main cause of AKI development in these patients. Hence, different strategies may be adopted depending on the level of “stress” to the kidneys on ICU arrival, which may be indirectly inferred by these simple parameters before significant increases in sCr.
Our study has several limitations. First, the nature of the analysis was retrospective which means that acquisition of the data was not originally done for the purpose of this study. In fact, a prospective study with simultaneous analysis of creatinine clearance as a surrogate of GFR is needed in order to confirm our findings. Second, we used widely available parameters both in blood and urine because we think that our findings must be applicable even in low resource ICUs; however, some of these parameters do not have a direct relation with AKI development. For instance, CRP is not directly implicated in AKI pathophysiology nor it is a specific marker of renal damage. Some studies have suggested that hypoalbuminemia may be considered as an independent risk factor for AKICitation22 but a causal relationship is not clearly confirmed. We believe that higher CRP and lower albumin are epiphenomena of the degree of SIRS especially because most cases were elective surgeries and the patients were not critically ill before surgery. Hence, even small increases in CRP and decreases in albumin at the immediate postoperative period may represent the level of the inflammatory insult which was just triggered by the procedure. Lower NaU may also be related to systemic inflammation as previously experimentally demonstrated.Citation23 On the other hand, we do not have the preoperative values of these parameters, so that we could not be sure that they were not already different between groups before surgery. ΔFEK as a valuable predictive parameter is questionable because most AKI were diagnosed at D1, the same day that ΔFEK was calculated. Nonetheless, it brought important insights in AKI pathophysiology since, in agreement with two previous studies,Citation6,Citation15 it demonstrates that the behavior of FEK is directly related to renal function remaining high (or even increasing) in AKI and low (or rapidly decreasing) in no-AKI. Finally, for the practical purpose of this study, D0 was considered the ICU admission and not the day of AKI diagnosis, a fact that might have reduced the homogeneity of the data of AKI patients in the subsequent days (e.g., a patient may have AKI resolving at D2 and another patient may have AKI diagnosed at D2). Some significant differences between AKI and no-AKI patients could have been minimized by such effect. Larger and prospective studies evaluating these same parameters but separating patients according to the postoperative day of AKI diagnosis would be appropriate in order to reveal if the severity of the changes in the studied parameters at ICU admission has some relation to the time until AKI diagnosis.
Conclusion
Acute kidney injury is a serious and early complication of major surgical procedures. Increases in sCr are usually observed in the next 2 days after ICU admission but lower NaU as well as lower FEUr and higher FEK values may sign for decreases in GFR even at ICU admission. Since most postoperative AKIs are triggered by an inflammatory insult, higher levels of CRP as well as lower serum albumin levels at ICU admission may alert for greater inflammation and, consequently, greater risk of AKI development. More complex physicochemical parameters seem to be of little help in this matter. Additional, prospective studies must confirm these preliminary findings and reevaluate these simple parameters as potential monitoring tools.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Romagnoli S, Ricci Z. Postoperative acute kidney injury. Minerva Anestesiol. 2015;81:684–696.
- Harris DG, McCrone MP, Koo G, et al. Epidemiology and outcomes of acute kidney injury in critically ill surgical patients. J Crit Care. 2015;30:102–106.
- Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib Nephrol. 2010;165:1–8.
- Schiffl H, Lang SM. Update on biomarkers of acute kidney injury: Moving closer to clinical impact? Mol Diagn Ther. 2012;16:199–207.
- Maciel AT, Park M, Macedo E. Physicochemical analysis of blood and urine in the course of acute kidney injury in critically ill patients: A prospective, observational study. BMC Anesthesiol. 2013;13:31.
- Maciel AT, Park M, Macedo E. Fractional excretion of potassium in the course of acute kidney injury in critically ill patients: Potential monitoring tool? Rev Bras Ter Intensiva. 2014;26:143–147.
- Kim DH, Shim JK, Hong SW, Cho KR, Kang SY, Kwak YL. Predictive value of C-reactive protein for major postoperative complications following off-pump coronary artery bypass surgery: Prospective and observational trial. Circ J. 2009;73:872–877.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236:579–581.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–2229.
- Elisaf M, Siamopoulos KC. Fractional excretion of potassium in normal subjects and in patients with hypokalaemia. Postgrad Med J. 1995;71:211–212.
- Maciel AT, Park M. Early diagnosis of acute kidney injury in a critically ill patient using a combination of blood and urinary physicochemical parameters. Clinics(Sao Paulo). 2012;67:525–526.
- Maciel AT, Park M, Macedo E. Urinary electrolyte monitoring in critically ill patients: A preliminary observational study. Rev Bras Ter Intensiva. 2012;24:236–245.
- Maciel AT, Nassar AP, Vitorio D. Very transient cases of acute kidney injury in the early postoperative period after cardiac surgery: The relevance of more frequent serum creatinine assessment and concomitant urinary biochemistry evaluation. J Cardiothorac Vasc Anesth. 2016;30:56–63.
- Prowle J, Bagshaw SM, Bellomo R. Renal blood flow, fractional excretion of sodium and acute kidney injury: Time for a new paradigm? CurrOpinCrit Care. 2012;18:585–592.
- Maciel AT, Vitorio D, Salles LD, Park M. Sodium concentration in urine greater than in the plasma: Possible biomarker of normal renal function and better outcome in critically ill patients. Anaesth Intensive Care. 2014;42:584–591.
- Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: A new perspective. Contrib Nephrol. 2010;165:39–45.
- Maciel AT, Vitorio D, Salles LD. Urine sodium profile in the course of septic acute kidney injury: Insights relevant for kidney function monitoring. Minerva Anestesiol. 2014;80:506–507.
- Santonocito C, De Loecker I, Donadello K, et al. C-reactive protein kinetics after major surgery. Anesth Analg. 2014;119:624–629.
- Rocktaeschel J, Morimatsu H, Uchino S, et al. Acid-base status of critically ill patients with acute renal failure: Analysis based on Stewart-Figge methodology. Crit Care. 2003;7:R60.
- Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. Intensive Care Med. 2010;36:1657–1665.
- Langenberg C, Wan L, Bagshaw SM, Egi M, May CN, Bellomo R. Urinary biochemistry in experimental septic acute renal failure. Nephrol Dial Transplant. 2006;21:3389–3397.