Abstract
It has been suggested that bone marrow derived stem cells have the ability to engraft the kidney and improve the outcome of severe acute kidney injury (AKI) in mice exposed to high doses of cisplatin, providing hope for cancer patients in whom irreversible renal damage occasionally occurs following the use of this highly effective anti-tumor drug. We tested the therapeutic potential of bone marrow derived cells injected during the acute phase (day 3 after cisplatin administration) of experimentally-induced AKI in C57Bl6/J mice, characterized by massive tubular necrosis, apoptosis, and a low proliferation capacity. We failed to show any benefit of bone marrow derived cells versus a regular homogenate of intact renal cells, or normal saline. Using cell tracers and flow cytometry, we demonstrated that bone marrow derived cells did indeed home to the bone marrow of the recipients but failed to settle in the kidney. Conversely, renal cells homed to injured kidneys. However, neither cell therapy protected the animals against cisplatin-induced death. We therefore question the short-term efficacy of bone marrow derived cells used to repair established injuries of the tubular epithelium.
Introduction
Platinum-based chemotherapy is widely used to treat solid cancers, such as testicular, bladder, cervical, and non-small cell lung cancers.Citation1 Cisplatin, the neutral cis-isomer of (PtII(NH3)2Cl2), was approved by the USA Food and Drug Administration in 1978, and is still widely used today. Its antitumor efficacy involves covalent binding with the purine bases of DNA (resulting in what are known as platinum-DNA adducts); this in turn activates a DNA repair process leading to apoptosis and eventually cell death.Citation1 Platinum is extensively bound to proteins, and so few free cisplatin molecules are filtered through the glomerular barrier; in addition, the tubules do not reabsorb luminal cisplatin. Nevertheless, cisplatin may still be taken up from the basolateral side of the tubular cells by organic cation transporters (OCT).Citation2 OCT2 in particular, is abundantly expressed by the S3 segment of the proximal tubules, which is located at the junction between the inner cortex and outer medulla. There, plastination of DNA induces tubular cell death and may lead to acute kidney injury (AKI). Nephrotoxicity is one of the main safety issues regarding the use of cisplatin, and has led to an extensive search for less toxic analogs that retain its antitumor efficacy.
For the past decade, ischemia reperfusion injury (IRI) has been used worldwide as an experimental model of AKI. Recently, sophisticated genetically-engineered mice have been used to help to elucidate how injured tubular epithelium is repaired after IRI. It turns out that the kidney has no interstitial stem cells, and consequently the repair of its tubular epithelium depends entirely on the presence of epithelial cells that have survived the injury.Citation3–5 It is interesting to note that unlike ischemic AKI—which has similar effects on the tubules in the outer cortex, but typically recovers in two to three weeks—cisplatin-induced AKI has a very variable prognosis, and occasionally leads to a permanent loss of kidney function.Citation6 This suggests that the extent of the DNA damage that has occurred makes regeneration impossible or ineffective.
In cancer patients, kidney transplantation is hardly an option: immunosuppressors would facilitate tumor growth, and the shortage of organs for transplantation makes cancer-free patients a priority. Repeated dialysis impairs quality of life, and survival is also compromised since much chemotherapy is contraindicated by persistent kidney failure. This makes the regeneration of native kidneys a question of life and death.
In the present study, we investigated the therapeutic interest of bone-marrow-derived stem cells (BMDC) during cisplatin-induced AKI. Our primary objective was to investigate the life-sparring benefit of BMDC injection at the acute phase of injury on the basis of three observations. First, genetically-intact, unsorted BMDC have been shown to repair genetically-induced glomerular diseases in mice by supplying the kidney with genetically intact cells,Citation7,Citation8 although this has been the subject of heated controversy.Citation9 Second, in mice BMDC were also found to prevent or reduce cisplatin-induced AKI through a paracrine action.Citation10–13 Third, in the clinic, stem cells are regularly harvested from cancer patients before lethal chemotherapy, to allow the repopulation of bone marrow by autologous cells and promote patient survival. We therefore reasoned that the administration of genetically-intact BMDC (i.e., in BMDC that had not been exposed to cisplatin) would be one way to regenerate the tubular epithelium following massive damage by platinum adducts. It was crucial to confirm their ability to home to the kidney and to test their efficacy during the acute phase of AKI, in order to parallel the pathology timeline observed in human patients.
Materials and methods
Ethics statement
Animal care and the experimental procedures were approved by an independent Ethics committee for animal experimentation (Comité National de Réflexion Éthique sur l’Expérimentation Animale, numéro 5) under the number Ce5/2012/004.
Animals
Eight-week-old female wild-type mice (C57BL/6J background, from 16 to 21 g of total body weight) obtained from Jackson Laboratory® (Bar Harbor, ME) and transgenic mice ubiquitously expressing green fluorescent protein (GFP) under the control of the promoter of β-actinCitation14 (C57BL/6-Tg (CAG-EGFP) C14-Y01-M131Osb) were maintained under standard conditions at the animal specific pathogen free facility located in the Research Building of Tenon Hospital; the mice were placed in 1284 L ventilated boxes, with a 12/12 photoperiod, at a 20–24 °C temperature and with 55 ±10% relative humidity. Food and water were available ad libitum. Experimentation begun one week after animal arrival.
Cisplatin
Cisplatin (MYLAN®, Canonsburg, PA, 1 mg/mL ) was further diluted in phosphate buffer saline (PBS) and administered as a single intraperitoneal injection containing a dose of cisplatin adjusted to allow for the bodyweight of the animal (doses of up to 20 mg/kg bodyweight were used to induce AKI, with an injected volume of 25 μl/g bodyweight).
Injection of the bone-marrow-derived cells
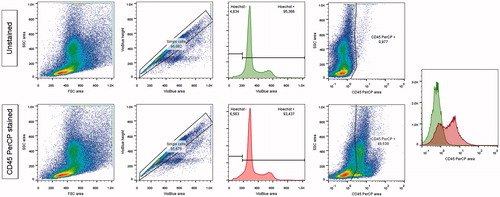
Briefly, the source animals for BMDC (whether wild type or GFP-transgenic) were sacrificed by cervical dislocation. Femurs, tibias and humeri were isolated. After section of the epiphyses, a diaphyseal flush with PBS was performed. A cell suspension was obtained after passage through 35-μm filters. After ammonium lysis of the red blood cells using a lysis buffer and potassium chloride (ACK), a flow cytometric cell count was performed. Three days after the injection of cisplatin, each mouse received a retro-orbital injection of 1 million BMDC diluted in 200 μl of sterile PBS, under isoflurane anesthesia. Using flow cytometry, we found that 51% [49; 59] of this global population were CD45 negative (); in comparison from 2 to 5 . 105 mesenchymal stem cells (MSC) had been used to rescue mice from cisplatin induced AKI in previous studies.Citation10–13
Figure 1. Flow cytometry analysis of bone-marrow, with a CD45 PerCP staining.
Analytic strategy: 1) size and structure of cells 2) inclusion of single cells comparing the height and area of the Hoechst signal (excitation by a violet laser at 405 nm and detection through a 450/50 nm filter) 3) inclusion of cells having a correct Hoechst signal (excitation by a violet laser at 405 nm and detection through a 425–475 nm filter) quantitative search for CD45+ cells in the PerCP channel (excitation by a laser at 488 nm and detection through a 655–730 nm filter).
It should be noted that the time between the flush and the orbital injection did not exceed an hour and a half. The BMDC were traced by detecting fluorescent GFP if the donor was GFP-transgenic, or detecting carboxy fluorescein succinimidyl ester (CFSE), after adding CFSE ex vivo (according to the Manufacturer’s instructions) when wild-type BMDC were being used.
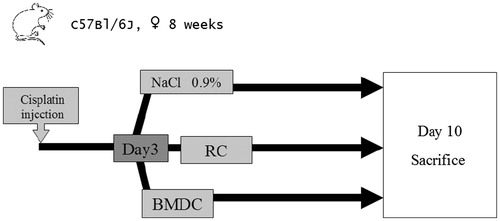
Two control groups were added: one was injected at the same time point with the same volume (200 μl) of isotonic saline (NaCl 0.9%), and the other, injected with a homogenate of renal cells from similarly syngeneic mice that had not been exposed to cisplatin (thus genetically intact but a priori devoid of stem cell properties), both also under isoflurane anesthesia (). To this end, after dermal ethanol asepsis, both kidneys were extracted, decapsulated, and stored in PBS under sterile conditions. A kidney cell suspension was obtained by passing the kidneys through a cell dissociation (04.1 Spleen protocol, gentle MACS™, Miltenyi Biotec®, Bergisch Gladbach, Germany), and filtering with a 35-μm filter. Here too, 200 μl of a solution containing one million cells was injected intravenously. The animals were syngeneic, and had not been subjected to irradiation before the injection of BMDC or renal cells.
Figure 2. Experimental protocol.
To rescue mice from cisplatin-induced acute kidney injury, 106 BMDC were injected on day 3 post cisplatin injection. Two control groups were used: one received 10Citation6 renal cells and the other received the same volume of normal saline. Survival was observed over 10 days, and then any survivors were sacrificed for organ study.
Mice were randomly assessed by blocks of five animals for orbital injection of BMDC, renal cells or normal saline and the person in charge of the injection was blind from either group. Renal failure per se is not painful: animals presented activity slowdown and signs of dehydration but did not require analgesic support, as approved by the Ethics committee for animal experimentation. To evaluate the primary outcome, animals were daily checked for spontaneous death as a direct result of the intervention. Surviving animals were sacrificed on day 10 post AKI, for blood collection under ketamine/xylazine anesthesia, and kidney and bone marrow retrieval after cervical dislocation.
Histological analysis
For light microscopy, the renal tissue was fixed in alcohol–formalin–acetic acid, embedded in paraffin, and cut into 4-μm sections. The slides were stained with Masson’s trichrome, periodic acid-Schiff and hematoxylin-eosin to assess tubular injury. Six random photographs were taken of each slide in non-overlapping cortical areas, at ×400 magnification, using an Olympus© (Tokyo, Japan) BX 51/DP70 microscope . The percentage of injured tubules was then scored semi-quantitatively (0: 0%, 1: < 10%, 2: 10%–25%, 3: 26%–75%, 4: > 76%), according to the presence of tubular dilation, the loss of the brush border, the presence of intra-luminal casts, and the loss of the epithelial border. For mice that survived for 10 days after the rescue injection of cells, a quantitative score was also used to look for subtle improvements: an 8 × 8 grid was superimposed over a representative picture of the renal cortex of each mouse. Fibrosis was quantified by Sirius Red staining under polarization contrast microscopy. In addition, immunohistochemistry was used to detect proliferating cells using the Ki-67 antibody from Abcam® (Cambridge, UK) (dilution 1/10,000) revealed by a rabbit secondary antibody from Histofine® (Nichirei Biosciences, Tokyo, Japan) and the DAKO® (Glostrup, Denmark) AEC kit, and apoptotic cells were revealed using the TUNEL (TdT-mediated X-dUTP nick end labeling) kit from Roche® (Basel, Switzerland) according to the manufacturer’s instructions.
Flow-cytometer analysis
The MACSQuant® Analyzer (Miltenyi Biotec , Bergisch Gladbach, Germany) was used. Flow cytometry was carried out at a distance from the sample. The kidneys were passed through cell dissociation (04.1 Spleen protocol, gentle MACS™, Miltenyi Biotec, Bergisch Gladbach, Germany) and then filtered (35 μm). The bone marrow was simply filtered (35 μm). The cells were then stored at −20 °C in a solution of PBS containing dimethyl sulfoxide (10%) and fetal calf serum (15%). EGFP fluorescence was searched for in the FITC channel (excitation by blue laser at 488 nm and detection through a 525/550 nm filter). Leukocytes were detected by an anti-CD45-VioBlue (Miltenyi Biotec, Bergisch Gladbach, Germany). To note, red blood cells from EGFP transgenic mice are not expected to express EGFP.Citation14 For each marker, one million cells in 100 μL were incubated for 30 min in the presence of the antibody under saturating conditions (5 μL), and then washed. Analysis was performed using FlowJo analysis software (Treestar Inc., Ashland, OR, version X.0.7). The analytic strategy involved including cell populations that were within the acceptable range of size and structure. Doublets were excluded by gating singlets after plotting the height versus the area of the forward scatter signal. Self-fluorescent events were excluded using a gating strategy on the VioGreen (Miltenyi Biotec, Bergisch Gladbach, Germany) channel (excitation by a violet laser at 405 nm and detection through 525/550 nm filters) in order to optimize the specificity of GFP detection.Citation15
Creatinine and urea analysis
Plasma creatinine and urea were measured using a Konelab automator (Thermo Fisher Scientific, Waltham, MA) with an enzymatic method (Konelab automator) and expressed in micro and millimoles per liter, respectively.
Real time PCR
RNA or genomic DNA (gDNA) was extracted from kidneys using TRI Reagent (Euromedex, Mundolsheim, France). After digestion with DNase I, the RNA was reverse transcribed with the Maxima RT Kit (Fermentas , Waltham, MA). The quality of cDNA and gDNA was assessed by the ratio of the absorbances at 260 and 280 nm. Both were then amplified by PCR in a LightCycler 480 (Roche Diagnostics, Basel, Switzerland) with SYBR Green (LightCycler 480 SYBR Green I Master; Roche Diagnostics, Basel, Switzerland) and specific primers for EGFP, IGF-1, β-glucuronidase (Gusb) and Actin b1 (Actb1) used as housekeeping genes (). EGFP primers were chosen on the basis of the Jackson Laboratory® (Bar Harbor, ME) genotyping procedure; those for Insulin Like Growth Factor-1 (IGF-1) and housekeeping genes were designed using the Universal Probe Library Roche website for operating under the following conditions: 95 °C for 5 min, 45 cycles at 95 °C for 20 s, 60 °C for 15 s, and 72 °C for 15 s (except for Actb1 PCR with gDNA: 30 s). Tissues from C57BL/6-Tg (CAG-EGFP) C14-Y01-M131Osb mice were used as positive controls.
Table 1. Primers used for qRT-PCR (mRNA strand sequences).
Statistical analysis
Data are given as medians (with 25th and 75th percentiles) for the quantitative variables. Comparisons were made using a Wilcoxon test, where appropriate. Survival was studied by Kaplan–Meier curves and log-rank tests were performed to compare groups. For comparison of quantitative variables between groups, a Kruskal–Wallis test was performed. All tests were two-sided and at a 0.05 significance level. Analyzes were performed using the R.2.14.1 statistical package (R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900,051-07-0, URL http://www.R-project.org/).
Results
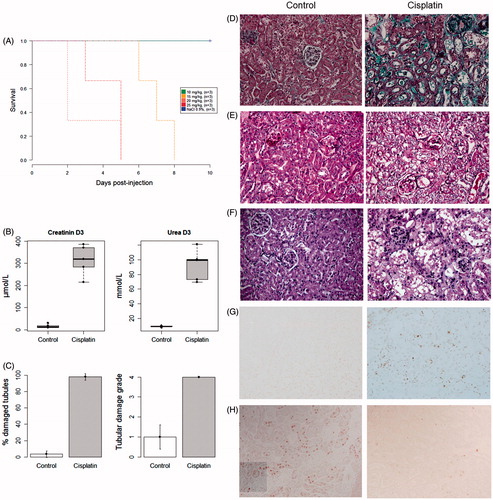
Cisplatin induced a dose-dependent mortality in C57Bl/6J mice (). Three days after injection of the cisplatin, renal function was severely impaired (), due to extensive tubular necrosis (). Massive apoptosis was observed (), together with a low ability of the epithelium to proliferate (). A single injection of 17.5 mg/kg of cisplatin was finally chosen to test the ability of BMDC to rescue the mice at day 3 (i.e., during the acute phase of tubular injury).
Figure 3. Lethality and severity of AKI following cisplatin injection.
A. Survival curve after the injection of increasing doses of cisplatin. B. Plasma creatinine and urea were increased three days after the injection of cisplatin (20 mg/kg). C. Mean percentage of injured tubules and corresponding histological damage scores at day 3. Masson's trichrome staining (D), periodic acid-Schiff staining (E), hematoxylin and eosin staining (F), showing the renal cortex of a mouse injected with saline (left) and that of a mouse injected with 20 mg/kg of cisplatin (right): tubular dilation, loss of brush border, the presence of intra-luminal casts and loss of the epithelial border were typical features of AKI. G. Detection of apoptotic cells using the TUNEL (TdT-mediated X-dUTP nick end labeling) assay. H. Detection of proliferating cells (Ki-67+) by immunohistochemistry. All photographs were taken after optical miscroscopy at ×400 magnification of the renal cortex.
Regarding the capacity of BMDC to prevent animal death after a lethal dose of cisplatin, 18/26, 13/26 and 16/25 animal died in the BMDC, renal cells and normal saline group, respectively. Survival curves show that both BMDC and renal cells failed to provide any significant benefit over saline (log-rank test p = 0.369) (). The kidneys of mice that survived for 10 days were all found to have similar tubular injury with some degree of regeneration (whether assessed by a semi-quantitative injury score: median value ± SEM for BMDC 3 ± 0.5; renal cells: 1 ± 0.3; saline 1 ± 0.5, p = NS; or by a quantitative injury score: mean values of 25.2 ± 8.0%, 8.0 ± 2.7% and 9.8 ± 6.5%, respectively, p = NS) (). No difference in renal functional parameters was found between groups () (p = 0.949 for plasma creatinine and p = 0.368 for plasma urea). No difference in interstitial fibrosis was found between groups (). The therapeutic groups displayed similar apoptosis (massive in each group), and similar capacities to proliferate (almost absent); no increase in IGF-1 transcripts was observed in mice injected with BMDC (p = 0.867) (). No major adverse event modified the experimental protocol.
Figure 4. Kaplan–Meier curve of mouse survival after cell rescue therapy.
Injection of 106 BMDC, 106 renal cells or of an equal volume of normal saline was performed three days after cisplatin injection. No difference in survival was found between the groups (log rank test, p = 0.369).
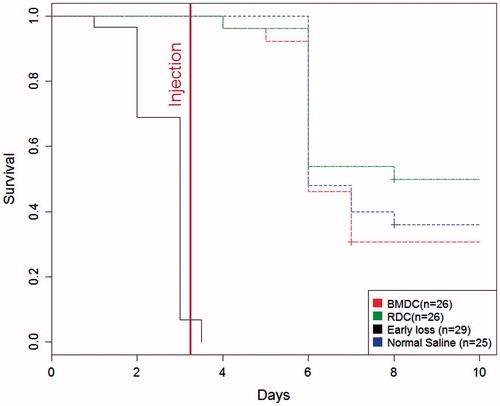
Figure 5. Histology of kidneys from mice which survived for 10 days after the rescue cell injection.
Analysis shows similar tubular injury with some degree of regeneration and similar interstitial fibrosis.
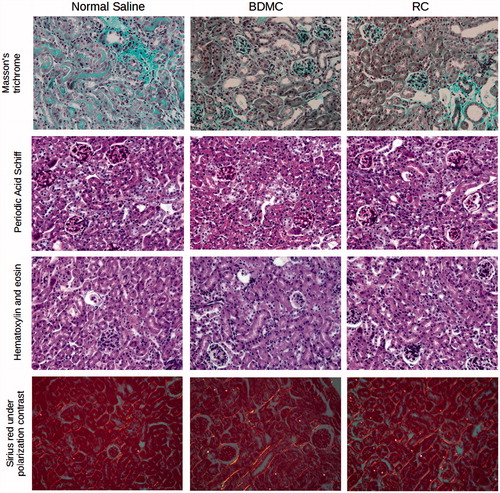
Figure 6. Renal functional parameters in surviving animals.
No difference in renal functional parameters was found between groups at day 10 in surviving animals (p = 0.949 for plasma creatinine and p = 0.368 for plasma urea).
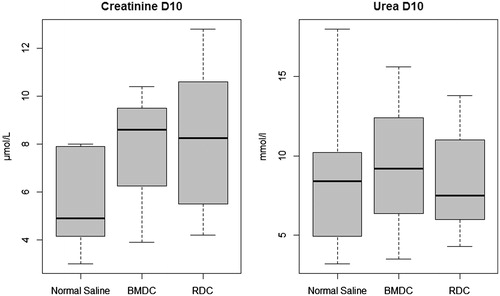
Figure 7. IGF1 messenger RNA real-time PCR.
No difference was found in IGF-1 transcripts among groups in the kidneys of mice that survived for 10 days.
Considering the absence of therapeutic effect, we reasoned that either the cells failed to home to the kidney or that cells were ineffective in rescuing AKI despite effective kidney engraftment. Cell homing was therefore assessed at day 10 among the surviving mice in blood and bone marrow, and in the kidney using flow-cytometry (). In the blood, we detected positive cytometric events both in the BDMC and the renal cells groups, where the number of positive events exceeded those of the control group in all individuals.
Table 2. Detection of labeled cells in recipient surviving mice by flow cytometry. Results are expressed as medians [Q1; Q3]. (*: BMDC vs. renal cells).
Regarding the bone marrow, a significantly higher proportion of positive events were detected in the BMDC group compared with the renal cells group (p = 0.006). The renal cells group showed a number of positive events equivalent to the negative control group. This result suggests a significant engraftment of BMDC in the recipients’ marrow and in contrast the absence of renal cells homing to the marrow. Analyzing the results obtained from the cell suspension of dissociated kidneys, there was a significant disparity in the results. A positive signal was observed in one individual from the control group, perhaps due to the constitutive autofluorescence of renal cells. One individual from the renal cells group showed a high proportion of positive cells (). The overall comparison of BMDC and RC groups, however, showed a significantly higher proportion of positive events in the renal cells group compared with the BMDC group (p = 0.012).
Figure 8. Flow cytometry analysis of renal cells homing to injured kidneys.
Analytic strategy: 1) inclusion of cell populations that were within the acceptable limits of size and structure; 2) exclusion of duplicates or aggregates by comparing the height and area of the signal; 3) self-fluorescent cells were excluded by gating non-fluorescent cells in the VioGreen channel (excitation by a violet laser at 405 nm and detection through a 525/550 filter); quantitative search for EGFP + cells in the FITC channel (excitation by a blue laser at 488 nm and detection through a 525/550 nm filter).
These results are to be understood taking into account differences in the analysis and gating used for cytometry. Indeed, kidney cells emit significant autofluorescence which requires a specific analysis. However, we failed to detect any BMDC by either direct immunofluorescence of kidneys or by PCR amplification of the EGFP transcript, whether we used RNA or gDNA extracted from kidneys (whereas the transgene was clearly amplified using RNA and gDNA templates from transgenic EGFP + tissues used as positive controls).
Discussion
Our study found no survival benefit following the use of genetically intact BMDC during the acute phase of cisplatin-induced AKI in mice, even though on four occasions an independent group has reported the contrary despite using similar amounts of stem cells, appropriate controls and a similarly severe model of AKI.Citation10–13 More precisely, MSC injected at day 1 were repeatedly found to protect mice against cisplatin-induced AKI (as opposed to hematopoietic stem cells, which had no therapeutic impact,Citation11 whether of mouseCitation10,Citation11 or human origin—thus both bone marrowCitation13 and cord bloodCitation12 human MSC gave successful outcomes.
Our experimental design was different, though: we injected BMDC during the acute phase of the disease (day 3), which is the context that confronts physicians at the bedside when a cancer patient develops a severe AKI following platinum-based chemotherapy. The animal model of cisplatin-induced AKI does not allow us to find out whether any benefit would be seen later on as dialysis is not an option in mice. The bulk of the mice did however survive for three days after the rescue cell injection, which was long enough for us to observe a difference in renal function,Citation11 and even survivalCitation12 in the experiments performed by Morigi and coworkers.
Apart from this difference, another difference should be pointed out. Morigi et al. used fluorescence technology (detection of the Y chromosome, or of a PKH-26 label) to trace stem cells and showed images of MSC localized in peritubular areas (and in a few cases inside tubules), in proportions from 0.5 to 3.4 per 105 renal cells.Citation10,Citation12 We failed to see any incorporation or even homing of BMDC in injured kidneys, even though we used highly sensitive techniques (flow cytometry and qRT-PCR) as well as two different fluorescent markers (GFP and CFSE), and of course a syngeneic background between donor cells and recipients.
With respect to the question of how MSC exerted their protective effect, it was initially argued that they might rapidly regenerate a damaged tubule through differentiation,Citation11 yet very low levels of intratubular incorporation and tubular differentiation were actually found. MSC seem to act mainly through their trophic effect, being a source of renotropic growth factors such as IGF-11.Citation10,Citation12,Citation13 The time point at which stem cells are injected to repair a tissue is undoubtedly critical. Under the experimental conditions that we used, BMDC did not affect survival at all, though, and we wonder to which extent this trophic effect is clinically relevant. We do not think that the severity of the model was an issue here, since we had a mean of 40% survival in each study group, while survival was null in the control group of the studies we are referring to.
A limitation of our study is that we used unsorted BMDC, and therefore a mixture of hematopoietic cells, mesenchymal stem cells, as well as lymphoid and myeloid progenitors. Although we are not aware that any of these components has any negative effect, we cannot exclude the possibility that one lineage could have canceled out the potential benefits afforded by MSC. However, this seems unlikely, because two independent teams have reported that unsorted BMDC display a protective role in the mouse model of Alport-like glomerular disease,Citation7,Citation8 although once again, these data have given rise to controversy.Citation9
In the context of organ injury, there is no evidence for the circulation of MSC in humans.Citation16 Injection seems to be necessary to provide a protective effect. Our study questions the homing capacity of cells as we did not find BMDC tagged cells in the injured kidney. Lastly, it is noteworthy that EGFP + renal cells were found to home in significant proportions to the injured kidneys and that this therapeutic group had the lowest histological injury scores. This finding supports the concept of kidney self-repair. Nevertheless, with respect to survival, which was our main outcome criteria, renal cells did not provide any significant protection.
In conclusion, our negative findings somewhat temper the hopes centered on cell therapy as a promising therapeutic option for cancer patients suffering from irreversible cisplatin-induced AKI. They also reinforce the concept that the epithelium itself is the major source of tubular regeneration in the context of severe tubular injury. Will stem cells fulfill their promise for patients in whom the renal epithelial reservoir has been exhaustively damaged by a toxic drug? Clinical studies are in progress which we are justified in hoping will resolve the question (Clinical Trials NCT0, 1275 612).
Acknowledgments
The authors thank Professor Masaru Okabe for providing the C57BL/6-Tg (CAG-EGFP) C14-Y01-M131Osb strain, Professor Selim Aractingi and Tatiana Ledent (INSERM UMRS 938) for their help in obtaining the GFP animals, Dr. Laurent Mesnard for his excellent technical skills in animal handling, and Ms. Caroline Martin and her team for animal care.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding information
This work was supported by the Fondation de l'Avenir, Paris, France [Project ET0-570], by the Region Ile de France (CORDDIM). AB and MW are the recipients of a fellowship “Année recherche” from the Agence Régionale de Santé (from Ile de France and Amiens, France, respectively). AH is the recipient of an Interface contract with the French National Institute for Health and Medical Research (INSERM).
References
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584.
- Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol. 2011;41:803–821.
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221.
- Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291.
- Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–9231.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460.
- Prodromidi EI, Poulsom R, Jeffery R, et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455.
- Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Nat. Acad Sci. 2006;103:7321–7326.
- Gross O, Borza D-B, Anders H-J, et al. Stem cell therapy for Alport syndrome: The hope beyond the hype. Nephrol Dial Transplant. 2009;24:731–734.
- Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928.
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804.
- Morigi M, Rota C, Montemurro T, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–522.
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082.
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. mice’as source ubiquitous cells. FEBS Lett. 1997;407:313–319.
- Legouis D, Bataille A, Hertig A, et al. Ex vivo analysis of renal proximal tubular cells. BMC Cell Biol. 2015;16:12.
- Hoogduijn MJ, Verstegen MMA, Engela AU, et al. No evidence for circulating mesenchymal stem cells in patients with organ injury. Stem Cells Dev. 2014;23:2328–2335.
