Abstract
Cancer is singled out as the biggest cause of death in the world, predicted to reach 13.1 million cancer-related deaths by the year 2030. Although there are no specific tumor markers used in cancer screening, some markers can be used to assist in making a diagnosis and determining a prognosis. They can be used to follow in cases where the diagnosis is cancer through monitoring of the disease recurrence and/or evaluating the response to therapy. These markers are not specific as the number increases in multiple cases of cancer. Some markers are positive in a single type of cancer; others are detectable in more than one type. An ideal tumor marker should be highly sensitive, specific, and reliable with high prognostic value. Other characteristics of an ideal tumor marker are organ specificity and correlation of it with tumor stages. However, none of the tumor markers reported to date has all these characteristics. Influence of different stages of chronic kidney function on serum tumor markers is variable. Furthermore, hemodialysis, peritoneal dialysis, and kidney transplantation affect on tumor markers differently. Sometimes, no study has been found in the literature review. Combined serum tumor markers may also be valuable. This literature review points the role of serum tumor markers in screening, diagnosis, and follow-up of cancer patients in chronic kidney disease patients and renal allograft recipients. In addition, impact of chronic kidney disease and kidney transplantation on different serum tumor markers is briefly explored.
Introduction
Cancer is one of the diseases which is found to be a major leading cause of death in worldwide due to the late diagnosis of the disease. Hence, early diagnosis of cancer plays a very important role in the management and the cure of the disease. Also, it has been known that besides the increased risk of cardiovascular mortality, chronic kidney disease is an important risk factor for cancer mortality. Patients with chronic kidney disease have a higher mortality risk of liver, kidney, and urinary tract cancers.Citation1 Therefore, early diagnosis of cancers with the help of serum tumor markers seems to be necessary. This paper is based on a systematic review of large literature and has been conducted through literature searches using medical subject headings that combined with terms for chronic kidney disease or dialysis or kidney transplantation. This also includes the method supplemented with author’s working knowledge and reference lists of review article and textbooks, and with references in articles that author found relevant.
Definition of serum tumor markers
Neoplastic cells secrete proteins that can mark their activities. The first tumor marker was discovered in 1847. However, now more than 100 different tumor makers are available. The serum tumor markers are proteins or glycoproteins which depending on their type are secreted by tumoral and normal cells. Tumor marker concentrations in healthy individuals is low or zero, and its increasing concentrations suggest the incidence of related tumor ().Citation2 Tumor markers are widely used in monitoring cancer patients and for screening of certain tumors. It has recently been shown that concentrations of some tumor markers are higher in patients with chronic kidney disease (CKD) than in healthy subjects (). Ideal tumor marker should be positive in patients only if malignancy is present or in progress, correlated with stage and response to treatment, repeatedly detectable, and easily measured. Unfortunately, such an ideal marker does not exist as yet.Citation3 Common tumor markers are described in the following sections.
Figure 1. Schematic presentation of various cancers that are associated with elevated tumor markers. Underlined cancers indicate primary tumors and others imply additional associated malignancies. Note: AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; HCG, human chorionic gonadotropin; PSA, prostate-specific antigen.
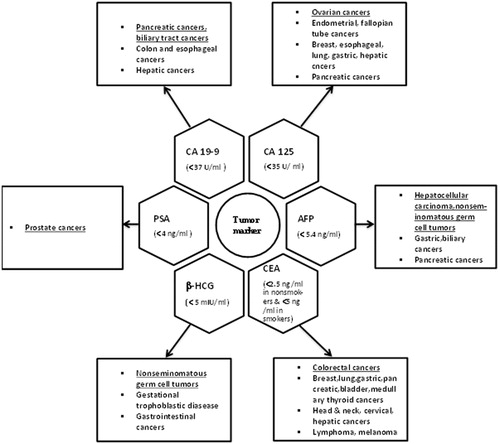
Table 1. Changes of serum tumor markers in NDD-CKD, hemodialysis, peritoneal dialysis, and renal transplant recipients.
Prostate-specific antigen in chronic kidney disease
The prostate-specific antigen (PSA) remains the best and most widely used tumor marker for prostate in clinical practice. PSA is a serine protease, which is produced by prostatic alveolar and ductal epithelial cells and it is a glycoprotein with a molecular weight of 33 kDa. PSA is commonly used in the diagnosis and follow-up of prostate disease especially prostate cancer. PSA was introduced into clinical practice in 1986, the early diagnosis and management of prostate cancer has been revolutionized. The PSA test is the most effective test that is currently available for the early detection, diagnosis, and follow-up of prostate cancer in kidney disease patients.Citation4
Prostate-specific antigen occurs in several molecular forms in the blood, with two predominant forms: free, non-complexed PSA (fPSA) with a molecular mass of ∼28 kDa and complexed PSA (cPSA), a stable ∼90 kDa complex with alpha-1 antichymotrypsin. PSA also occurs in complex with alpha-1 antitrypsin and alpha-2 macroglobulin but at much lower levels and of certain clinical value. The sum of fPSA and cPSA roughly corresponds to the conventional immunodetected total PSA (tPSA). Increased release of tPSA in the blood is caused by prostate cancer but also by other prostate disorders, such as benign enlargement and prostatitis. However, fPSA as a percentage of tPSA (percent fPSA) is lower in men with prostate cancer than in men with benign disorders. Half-life for fPSA in serum is short (4-34 h). This, combined with its low molecular mass, suggests elimination by glomerular filtration. In contrast, cPSA has slow elimination kinetics and 3-fold larger molecular mass, which prevents glomerular filtration; these observations suggest other routes of elimination, most likely by liver metabolism. Therefore, diminished renal elimination of fPSA may affect percent fPSA and its accuracy as a diagnostic tool for prostate cancer. In a study by Bruun et al.,Citation5 101 men with chronic kidney disease without prostate cancer and also 5264 men with no diagnosis of prostate cancer during 8 years follow-up were considered. With adjustment for age, median fPSA levels and percent fPSA were significantly higher in patients with renal dysfunction. The percent fPSA is importantly influenced by moderately impaired renal function in men with chronic kidney disease. For such men, use of the current clinical decision limits for percent fPSA could cause some men with prostate cancer to be misdiagnosed as having benign disease, and therefore fPSA should not be used to diagnose prostate cancer in these patients. Impaired renal function is thus an important factor to consider in the evaluation of percent fPSA for detection of prostate cancer.
Prostate-specific antigen in chronic dialysis
Both hemodialysis and peritoneal dialysis are believed to influence the PSA-based results and several studies have established these findings ().Citation6–15 Renal dysfunction may alter the relative proportions of the two PSA forms by decreasing the elimination of fPSA, thereby increasing percent fPSA. This has been confirmed by findings in men on hemodialysis and peritoneal dialysis treatment who had significantly higher percent fPSA. This raises the possibility that renal dysfunction may compromise the diagnostic accuracy of percent fPSA and suggests that a high percent fPSA could not be considered as a sign of benign prostatic disease in men with strongly reduced glomerular filtration rate (GFR) requiring chronic dialysis.Citation5
Figure 2. Different studies of prostate specific antigen (tumor marker) in chronic kidney disease. Note: AFP: alpha-fetoprotein; CAPD: continuous ambulatory peritoneal dialysis; CKD: chronic kidney disease; %fPSA: percent free prostate-specific antigen; HCG: human chorionic gonadotropin; HD: hemodialysis; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density.

Prostate-specific antigen is a tumor marker that is used as a screening test, usually in combination with digital rectal and/or ultrasound-guided examination. Evaluating tumor markers in dialysis patients can be difficult but total PSA is probably valid in patients with end-stage renal disease because total PSA is not cleared by dialysis membranes. Free PSA and free/total PSA ratios are reliable only with low-flux membranes. Free PSA in dialysis patients are less useful because free PSA tends to be elevated in association with hemoconcentration and its clearance is affected by high-flux dialysis membranes. Normal % free PSA do not apply to dialysis patients.Citation14,Citation15
Prostate-specific antigen in kidney transplantation
Cancer is the second to cardiovascular disease as one of the major causes of morbidity and mortality in renal transplant recipients. Cancers can recur, occur de novo, and be transmitted from donor organ in post-transplantation period. Renal transplant recipients (RTR) are at high risk for de novo cancers, especially skin cancer and post-transplant lymphoproliferative disorder (PTLD) with an incidence 4- to 20-fold higher than that in the general population. Genito-urinary (GU) malignancies have been reported to be the second most common malignancies in the RTR population in the United States. Prostatic cancer in renal transplant recipients is two- to five-fold higher than that in the general population. Kleinclauss et al.Citation16 in a retrospective, multi-centre study examined 62 patients in renal transplant recipients. This study highlights particular characteristics of prostate cancer in the RTR population. First, prostate cancer appears sooner in RTR than in the general population. Second, they observed a high rate of advanced or metastatic disease and third, the stage of the disease seems to be related with the immunosuppressive therapy (calcineurin inhibitors and azathioprine) usage. Hence, a systematic screening of prostate cancer is required in renal transplant recipients by an annual serum PSA level and direct rectal examination.Citation15–17 In a study by Mahdavi et al.,Citation18 40 patients who were on peritoneal dialysis or hemodialysis underwent kidney transplantation. Free PSA levels were significantly decreased after kidney transplantation but total PSA remained unchanged. This was observed in post-transplant patients irrespective of whether they had delayed graft function, slow graft function, or immediate graft function during the observed period. Therefore, total PSA can be used as a marker for prostate cancer screening after kidney transplantation. Other interesting points about prostatic-specific antigen contain situations that may change PSA values like inflammations, traumatic interventions, and immunosuppression. Chamie et al.,Citation19 in their study concluded that only immunosuppression with sirolimus independently impacted post-transplant PSA. Their data strongly suggest that sirolimus is associated with a significant decreased level of PSA in kidney transplantation.
CA 15-3 and CA 27.29 in chronic kidney disease
CA 15-3 (also known as MUC1 or EMA) is the serum marker most widely used in breast cancer. Like CA 125, MUC1/CA 15-3 is a member of the mucin family and is a membrane-associated O-glycoprotein with a large extracellular domain. CA 15-3 is expressed on the apical membrane of almost all glandular epithelia, and in normal kidney it is localized in the distal convoluted tubules and in the collecting ducts. CA 15-3 has a significant role in cell adhesion and cellular polarity and through its intracellular domain is implicated in signal transduction, interacting with the epidermal growth factor (EGF) receptor, and activating the mitogen-activated protein (MAP) kinase pathway.Citation20 CA 15-3 is structurally a glycoprotein that is principally detected by immunoabsorption and may be elevated in patients with breast, ovarian, prostate, and lung cancer and is also reported to be increased in hepatitis conditions, benign breast or ovarian disease, endometriosis and pelvic inflammatory disease.Citation21
CA 15-3 and CA 27.29 are, substantially, the same molecule but measures of different epitopes of the same protein antigen product of the MUC1 gene. Normal serum levels of CA 15-3 and CA 27.29 are less than 31 and 38 units/mL, respectively. Serum levels of CA 15-3 and CA 27.29 are not useful for breast cancer diagnosis, but their use is recommended in metastatic breast cancer treatment, since they reflect the course of disease in 75 to 90% of patients undergoing therapy. However, the routine use of serial tumor measurement of these markers in the post-treatment surveillance of women with breast cancer is controversial, particularly in patients with chronic kidney disease and renal replacement therapy.Citation3
CA 15-3 in chronic dialysis and kidney transplantation
In a study by Tzitzikos et al.,Citation22 30 patients on intermittent hemodialysis and with clinical-free neoplastic disease to determine the reliability of the serum markers, CA 15-3, CA 19-9, CA 125, alpha-fetoprotein and CEA were selected. CA 15-3 levels were twice normal in four (13%) patients. More than half (7/13) of anti-HCV positive and all of Australian antigen positive patients had abnormal serum levels of CA 15-3 and CA 125 after hemodialysis treatment. However, the elevated levels of other markers including CA 15-3 and CA 125 are not specific for neoplasms and are related to active hepatitis C. Another study by Estakhri et al.,Citation2 100 patients were selected for the study to investigate differences between serum levels of tumor markers CA 15-3, AFP, CA 19-9 and CEA in patients with impaired renal function. A significant correlation was observed between serum levels of AFP and CA 15-3 and also a tendency between creatinine clearance and CEA. Creatinine clearance significantly correlated with AFP and CA 15-3, but not CA 19-9. Therefore, for the diagnosis and follow-up of cancer patients with kidney failure by AFP, CA 15-3 should be taken with the necessary precautions and if possible, another more reliable method that is not affected by renal failure can also be used. Moreover, in a study by Xiaofang et al.,Citation23 232 non‐dialysis patients with CKD and 37 uremic patients on maintenance hemodialysis were enrolled. The serum concentrations of CA 19‐9, CA 125 (male), cytokeratin fragment 21-1 (CYFRA 21‐1), neuron-specific enolase (NSE), and squamous cell carcinoma (SCC‐Ag) correlated negatively with creatinine clearance, while there were no significant differences in the concentrations of CA 125 (female), AFP, CA 15-3, and CA 72.4. The serum levels of CA 19‐9, CA 125, AFP, CA 15-3, CA 72.4, CYFRA 21‐1, NSE and SCC‐Ag showed no differences between hemodialysis patients and non‐dialysis controls. The increase in the serum levels of CA 19‐9, CA 125 (in males), CYFRA 21‐1, NSE and SCC‐Ag in patients with CKD affects the specificity of these markers in the diagnosis of cancer. Hemodialysis does not affect the serum levels of CA 19‐9, CA 125, AFP, CA 15-3, CA 72.4, CYFRA 21‐1, NSE, and SCC‐Ag.
CA 19-9 and CA 50 in chronic kidney disease
Carbohydrate antigen 19-9 (CA 19-9) is a 36-kDa glycolipid detected in low levels in serum of healthy individuals. It is used as a marker for biliopancreatic malignancies and levels of it can be increased with other types of cancer, including gastric, colorectal, hepatic, ovarian, and transitional cell carcinoma. CA 19-9 levels can also increase in cases of biliary obstruction of non-malignant origin, as well as in benign hepatobiliary conditions, in which it correlates with serum bilirubin concentration. Increased serum CA 19-9 levels have also been reported with other non-malignant conditions. Levels of greater than the upper limit of normal are found in 0.6-0.8% of healthy individuals.Citation24 Appreciable concentration of CA 19-9 is also present in mucin rich saliva, seminal fluid, gastric juice, amniotic fluid, urine, ovarian cyst fluid, pancreatic, gall bladder, and duodenal secretions. In 99.6% of healthy adults, serum CA 19-9 levels are lower than 37 unit/mL. Value less than 100 U/mL is considered as grey zone values in which malignant and benign diseases may overlap. Malignant tumors in values above 100,000 U/L may be observed. CA 19-9 is neither tumor specific nor organ specific. However, the diagnostic sensitivity (85%) and specificity (95%) of CA 19-9 are highest for the adenocarcinoma of pancreas.Citation25 Moreover, the diagnostic performance of the biomarker is closely related to tumor size. Serial monitoring of CA 19-9 levels is useful for follow-up of patients after surgery and those who are receiving chemotherapy for advanced disease. In different studies, such as Zeferos et al.’s,Citation26 tumor markers of AFP, CEA, CA 19-9, CA 125, and CA 15-3 were studied in 50 healthy volunteers (group A), in 23 patients on chronic hemodialysis (group B), and in 30 successfully transplanted individuals (group C). The levels of CA 19-9 and CA 125 did not differ significantly among the three groups. CA 19-9 and CA 125 can be considered as reliable tumor markers in patients undergoing hemodialysis or kidney transplantation. In a study by Xiaofang et al.,Citation23 the increase in the serum levels of CA 19‐9, CA 125 (in males), CYFRA 21‐1, NSE and SCC‐Ag in patients with CKD affects the specificity of these markers in the diagnosis of cancer. Hemodialysis does not affect the serum levels of CA 19‐9. Oberbauer et al.,Citation27 the specificity and the sensitivity of tumor markers of AFP, CA 19-9, CEA, CA 125, CA 15-3, PSA, and calcitonin in 575 renal allograft recipients were evaluated. Impaired liver function was associated with significantly elevated AFP, CA 19-9, CA 125, and CA 15-3 levels. Finally, routine screening of the transplant population with serum tumor markers was not useful because of the low sensitivity and specificity of these tests. In addition, studies by Estakhri et al.Citation2 and Tzitzikos et al.Citation22 showed that CA 19-9 with presence of increased levels is not specific for neoplasms after hemodialysis treatment. Wood et al.Citation28 concluded that single isolated determinations of CA 125 and CA 19-9 are not reliable indicators of malignancy in renal transplant patients. Therefore, CA 19-9 and CA 50 (similar in structure to CA 19-9) should be interpreted with caution, often elevated in dialysis patients but has good specify and of some clinical value, often moderately elevated with peritoneal, pleural, or pericardial fluids.Citation15,Citation21,Citation25
CA 125, HE4, and CA 72.4 in chronic kidney disease
CA 125 (cancer antigen 125 or carbohydrate antigen 125), a tumor-associated glycoprotein of more than 200 KD.CA 125 (also known as MUC16) is a high molecular weight glycoprotein used mainly as a diagnostic biomarker for ovarian cancer. Elevated levels of this protein have also been found in tumors of the pancreas, lung, breast, and bladder, as well as in different benign diseases.Citation3,Citation20,Citation25 It is a component of the ocular surface, respiratory tract, and female reproductive tract epithelia. Normal values range from 0 to 35 unit/mL. CA 125 levels may be higher in premenopausal than postmenopausal women and half-life of it is approximately, 5-7 days.Citation29 In patients with elevated CA 125 levels, changes in biomarker levels have tracked tumor burden with greater than 90% accuracy. Persistent elevation of CA 125 following chemotherapy has correlated with residual ovarian cancer in >90% of cases, leading to approval of CA 125 by the United States Food and Drug Administration (FDA) for detection of disease that has survived primary chemotherapy. Monitoring CA 125 levels in patients with a complete clinical response can detect recurrence of cancer in up to 70% of patients, with an average lead time of 3 to 4.8 months. The clinical value of early detection of disease recurrence has been questioned, that is based on a single study with significant limitations, but it does provide time for patients to receive multiple conventional drugs and to participate in clinical trials with novel agents. Human epididymis protein 4, or HE4, was discovered by Kirchhoff and associates in 1991 and belongs to the “four disulfide core” family of proteins, which typically function as proteinase inhibitors. HE4 promotes migration and adhesion of ovarian cancer cells and in in vitro studies, HE4 knockdown resulted in tumor growth inhibition. HE4 may have an additional role in maintaining the innate immunity of the respiratory tract and the oral cavity. Various factors aside from malignancy may influence serum HE4 levels and should be carefully considered in interpreting values of HE4. Unlike CA 125 levels, which decrease with age, HE4 levels increase significantly with age. HE4 levels are also affected by pregnancy: pregnant women have significantly lowered levels of HE4 in comparison with age-matched non-pregnant premenopausal women. Older women, women with a later menarche, and smokers had also significantly higher levels of HE4 compared with appropriate controls. Levels of CA 125, Carcino-embryonic antigen (CEA), squamous cell carcinoma antigen (SCC), Neuron specific enolase (NSE) can be falsely elevated and have to be interpreted with caution. Levels of CA 125 are also altered in patients on continuous ambulatory peritoneal dialysis (CAPD) and have been noted to rise in peritonitis and immediately after implantation of the catheter.Citation30 In a study by Engin et al.,Citation21 levels of CA 125 were higher in CAPD patients than in the control and other groups. Aside from these factors, menstrual cycle, endometriosis, estrogen and progestin contraceptive usage do not alter serum levels of HE4. HE4 has emerged as a valuable biomarker for both ovarian and endometrial cancer. HE4 has been evaluated for distinguishing malignant from benign pelvic masses, prognostication, monitoring, and screening. Elevation of HE4 can occur in renal failure and in lung cancer.Citation31 HE4 is a superior biomarker for distinguishing benign from malignant gynecological disease. A combination of CA 125 and HE4 was found to be a better predictor of malignancy than either marker alone. This combination was also found to have diagnostic relevance in the setting where there is a need to distinguish endometrial cancer from benign uterine disease, with a sensitivity of 60.4% and a specificity of 100%. Using a combination of HE4 and CA 125 increased overall sensitivity to 76%. A combination of CA 125 and HE4 may offer better lead times and sensitivity for the detection of recurrent ovarian cancer.Citation32 HE4 levels may also aid in monitoring response to therapy. A combination of sophisticated imaging techniques combined with HE4 marker levels may aid in following patients with peritoneal carcinomatosis.Citation33
Cancer antigen 72.4 (CA72.4), a glycoprotein, which increases in gastric, colon, breast, and ovarian adenocarcinomas, may be employed alone or in combination with CA 125 and HE4. CA 72.4 is less sensitive than CA 125 for epithelial ovarian carcinoma (EOC), but it is not influenced by pregnancy or the phase of menstrual cycle.Citation34 Yontem,Citation35 in a research, investigated 24 patients with CKD on hemodialysis without malignancy and 24 people as the control group. In both groups, blood sample for serum tumor markers of CEA, CA 125, CA 15-3, and AFP were analyzed. There were no significant difference between the results of AFP and CA 125 of both groups. Therefore, serum tumor markers may increase in the patients with chronic kidney disease due to damaged renal clearance and elimination without malignancy. There were no significant differences in the concentrations of CA 125 (female), AFP, CA 15-3, CA 72.4. The serum levels of CA 19‐9, CA 125, AFP, CA 15-3, CA 72.4, CYFRA 21‐1, NSE, and SCC‐Ag showed no differences between hemodialysis patients and non‐dialysis controls. Hemodialysis does not affect the serum levels of CA 19‐9, CA 125, AFP, CA 15-3, CA 72.4, CYFRA 21‐1, NSE, and SCC‐Ag.Citation2,Citation22,Citation23,Citation26,Citation27 CA 125 serum levels are not influenced by kidney transplantation.
Afamin and apoA-IV are expressed predominantly in liver and small intestine, respectively. It will also be interesting to look at possible accumulations of both proteins in the ascitic fluid of the tumor and other properties of these proteins, such as anti-oxidative properties. Afamin accounts as an adjunct marker to CA 125 in ovarian cancer.Citation36 So far, no study has performed about Afamin in chronic kidney disease. The human glycoprotein of Afamin is primarily of hepatic origin and abundant concentrations are found in plasma, cerebrospinal fluid, ovarian follicular, and seminal fluid. Afamin is strongly associated with metabolic syndrome.Citation37 In a study by Freue et al.,Citation38 plasma concentration of Afamin for diagnostic purposes in patients with biopsy-confirmed acute rejection (BCAR) were measured and results revealed decreased levels of Afamin in blood. This study provides evidence that protein concentrations in plasma may provide a relevant measure for the occurrence of BCAR and offers a potential tool for immunologic monitoring.
Alpha-fetoprotein
Alpha-fetoprotein (AFP), a very popular and extensively studied carcinoembryonic glycoprotein/Oncofetal antigen, is a major fetal serum globulin with a molecular weight of approximately 65,000. The single chain of glycoprotein has carbohydrate content of 3% and amino-acid sequence similar to albumin. It is expressed during malignancy or during intra-uterine or early post-natal life. It is found in fetal liver, yolk sac, and the gastrointestinal tract. Consequently, AFP correspondingly increased in about 80% of patients with hepatoma, 60% patients with non-seminomatous germ cell cancers, and occasionally in patients with other cancers. Although, abundant in fetal blood, its concentration in normal adults is below 15 ng/mL. The appearance of excess amount of serum alpha fetoprotein beyond 500 ng/mL indicates underlaying malignancy except in the cases of pregnancies.Citation21,Citation25 Serum AFP measurement is a valuable clinical aid in diagnosis, prognosis, and monitoring of primary hepatocellular carcinoma, hepatoblastoma, non-seminomatous testicular germ cell tumors, the embryonal carcinoma, teratoma, choriocarcinoma and yolk sac carcinoma, germ cell tumors of ovary, and extra-gonadal germ cell tumors. Approximately, 60-90% patients with primary hepatocellular carcinoma have serum AFP concentrations more than 500 ng/mL. Very significant elevation of serum AFP is documented rarely in malignancies of gastrointestinal tract, pancreas, lung, kidney, breast, and etc. Regenerated hepatic tissue following liver damage due to viral hepatitis, chemically induced necrosis, and liver surgery have also associated with moderately raised AFP levels. An increase in serum AFP concentration below 400 ng/mL was also reported in 10-15% cases of acute and chronic hepatitis, liver cirrhosis, and secondary hepatic malignancies. Serial AFP estimations help in distinguishing non-malignant and malignant conditions, as steady and progressive rise of AFP is observed in malignancies, whereas non-malignant conditions show fluctuations and transient moderately elevated concentrations. Measurement of serum AFP has been helpful as laboratory aid in diagnosis and prognosis of AFP secreting malignancies, it has also been useful in monitoring efficacy of chemotherapy, surgery, and radiotherapy in primary hepatocellular carcinoma, hepatoblastoma, non-seminomatous testicular, and other germ cell tumors. AFP should not be used for tumors other than hepatocellular carcinoma, hepatoblastoma, and NSGCT. In particular, AFP should not be used for metastasis to liver. Half life of AFP in serum is approximately 5 days.Citation29 In many studies, the serum levels of AFP have been shown to be unaltered in patients with CRF, ESRD, hemodialysis, peritoneal dialysis, and renal transplantation.Citation2,Citation3,Citation15,Citation21,Citation35
Beta2-Microglobulin
In 1968, Berggard and Bearn isolated β2-microglobulin (β2m). It is a 100 amino-acid single polypeptide chain, with a globular structure maintained by a disulfide bond linking two cysteines in position 25 and 80, respectively.
It has a molecular weight of 11,815 Da and a stokes radius of 16 angstrom. It is found at the surface of human cells expressing major histocompatibility (MHC) class I.Citation39 β2-M is a low molecular weight protein and is part of the human leukocyte antigen (HLA) molecule, representing invariant light chain. It exists in the membrane of almost all nucleated cells, and is detectable in all body fluids as a shedding product of the cell membrane. The levels of serum and urine β2-M are also found to be increased in patients with some tumors, including solid tumors and leukemia. Thus, the levels of serum β2-M have become one of the most important prognostic factors and predictors of survival in patients with some tumors.Citation40 The role of β2-M has been demonstrated in several solid cancers and leukemia; however, the mechanism of β2-M action is poorly understood. Although increased β2-M serum levels in patients with breast cancer have been previously reported, the clinical value of β2-M as a prognostic factor and predictor of survival and also its mechanism in patients with breast cancer need further study, since breast cancer has different molecular subtypes and patients with the same clinical stages and pathological types of breast cancer that treated with same scheme, have different therapeutic and prognostic effects. In addition to the roles of β2-M in immunity, several other β2-M functions with clinical relevance have been elucidated, including the regulation of survival, proliferation, metastasis, and even apoptosis of cancer cells. Elevated β2-M has been observed in patients with renal failure and autoimmune and infectious diseases. Furthermore, increased synthesis and release of β2-M occurs in several malignant diseases including multiple myeloma, lymphoma, and solid tumors as indicated by elevated serum or urine β2-M concentrations. Also, level of β2-M is one of the most important independent prognostic factors and survival predictors for some cancers.Citation41 In diseases with an activated immune system and in hematologic cancers (leukemia, lymphoma, and myeloma), the β2-M synthesis in serum is enhanced. β2-M is filtered by glomeruli and catabolized in the tubuli. Serum β2-M concentrations are correlated inversely to residual renal function. Although serum levels of β2-M in healthy persons range between 1.5 and 3 mg/L, concentrations of 20 to 50 mg/L are found in patients with ESRD. β2-M concentrations increase with chronic kidney disease stage and are highest in hemodialysis (HD) patients. One study showed that renal transplantation, which reduced β2-M serum levels, inhibited the development of dialysis-related amyloidosis and alleviated its symptoms. β2-M is considered as a marker for dialysis adequacy of molecules in the middle molecular weight range.
β2-M was found to promote the growth of human renal cell carcinoma through activation of the protein kinase A, cyclic adenosine monophosphate (AMP) - responsive element-binding protein and vascular endothelial growth factor axis. Overexpression of β2-M in human prostate cancer cell lines leads to inhibition of tumor growth in vivo and using the β2-M antibody to interrupt β2-M signaling in human prostate cancer cell lines inhibits cancer cell growth and induces cell apoptosis.Citation42 Another study speculated that the up-regulation of β2-M expression may contribute to the oncogenesis of human oral mucosa, tumor invasion, and metastasis.Citation43 Other interesting point about β2-M is that expression ratio of Map7/β2-M may serve as a valuable prognostic marker in patients with Stage II colon cancer, and potentially guide therapeutic decision making.Citation44
As β2-M is cleared by the kidneys, β2-M levels also reflect renal function. In addition, as β2-M may also be part of the acute-phase response, non-specific elevation in β2-M may occur as a result of other immune stimulation, such as acute viral infection. β2-M is expressed in human prostate cancer cell lines and tissues. Serum β2-M levels are elevated in patients with metastatic, androgen-independent prostate cancer. β2-M is a secreted protein expressed in prostate cancer, which is more specific for androgen stimulation.Citation45 In a study performed by Rodriguez et al.,Citation46 201 patients to investigate the prognostic significance of serum β2-M levels among patients with chronic myelogenous leukemia (CML) were enrolled. Serum β2-M levels are an important, and probably independent, prognostic factor for patients with CML in early chronic phase that are treated with interferon (IFN)-based therapy. β2-M has been advocated as a better predictor of glomerular filtration rate (GFR), but its serum concentrations can increase as an acute-phase reactant in disorders, such as lupus nephritis. It has been shown that β2-M may be a more sensitive marker of renal function than serum creatinine, but it is overtly affected by non-renal factors. β2-M may be useful as a renal marker in specific situations, such as renal transplant monitoring.Citation47 However, the mean predialysis β2-M concentration over time was predictive of all-cause mortality, independent of the dialyzer β2-M clearance and the residual renal function. Higher β2-M levels correlate with various cardiovascular risk factors and inflammation markers, such as C-reactive protein (CRP), interleukin-6 (IL-6) and tissue necrosis factor (TNF-α) and are associated independently with cardiovascular mortality and cardiovascular events. However, when the oxidative burst of leukocytes was investigated, β2-M did not show proinflammatory properties and therefore may not by itself be a causative factor of vascular damage. The serum β2-M level is also a new predictor of diabetes-related mortality in diabetic patients irrespective of renal function and is associated positively with insulin resistance. Successful kidney transplantation results in a substantial decrease in β2-M levels, but a delayed decrease or subsequently increasing levels after transplantation may serve as a marker of acute rejection or inflammation, including cytomegalovirus infection and post-transplant lymphoproliferative disease. However, the relevance of post-transplant β2-M levels for long-term prognosis is unknown. A retrospective cohort study showed that serum β2-M at discharge is a potent predictor of long-term mortality and graft loss in kidney transplant recipients, providing information on allograft function beyond serum creatinine.Citation48 Elevated β2-M levels are observed in chronic renal failure, lymphoproliferative disorders, inflammations, infections and other conditions with high cell turnover. A relationship has been noted between tumor burden and β2-M. Concerning its use in oncology, β2-M levels correlate with the disease stage and poorer prognosis in patients with multiple myeloma and chronic lymphocytic leukemia. It is also the most important predictor of treatment-free survival and overall survival of patients that affected by lymphocytic leukemia and in most cases of lymphatic neoplasia.Citation49 Based on the findings of a study by Raikou and Kyriaki,Citation50 they investigated that elevated serum concentrations of β2-M predispose to an up-regulation of the inflammatory procedure, which is associated with the increased glucose serum concentrations in dialysis patients. On the other hand, the elevated glucose serum concentrations were found as a strong determinant of β2-M serum levels in dialysis patients, thus a better glycemic control in combination to a better β2-M clearance that obtained by a good dialysis adequacy should be very beneficial for this population of patients and resulting in down-regulation of inflammation. β2-M downstream signaling regulates androgen receptor (AR) and PSA expression directly in AR positive prostate cancer cells. In both AR-positive and AR-negative prostate cancer cells, interrupting β2-M signaling with the β2-M antibody inhibited cancer cell growth and induced its apoptosis. The β2-M antibody is a novel and promising therapeutic agent for the treatment of human prostate cancers.Citation51
The usefulness of β2-M as tumor marker is limited in the presence of CKD, since the impairment of renal function may increase β2-M levels in an unpredictable way. In this case when using β2-M as a tumor marker, it is appropriate to adjust its values for the degrees of deterioration of renal function calculated with GFR.Citation3
Human chorionic gonadotropin in chronic kidney disease
Human chorionic gonadotropin (HCG), a marker of germ cell tumors and trophoblastic disease, is 45KD glycoprotein, composed of two dissimilar subunits of the alpha chain (14 KD) and beta chain (24KD). It contains 30% carbohydrate. The beta subunit (β-subunit) determines immunological and hormone specificity. HCG is synthesized by the syncytiotrophoblasts of the placenta during pregnancy. The reference values in serum of healthy men and non-pregnant women are less than 5 IU/L and post-menopausal women are less than 10 IU/L. HCG shows 100% sensitivity for choriocarcinoma irrespective of their site in addition to hydatidiform mole. In testicular tumors, the detection of HCG and AFP correlates with the histological findings, and is therefore crucial for the therapeutic procedures with the use of serial determinations of β-HCG, because the biochemical recurrence precedes by 3 months before the patient has symptoms of clinical recurrence/metastases. The tumor marker also helps in monitoring high-risk group of testicular tumors especially individuals with undescended testicle or the healthy monozygotic twin of a testicular tumor patient. High levels of β-HCG indicate poor prognosis and frequent assays of HCG level during therapy are correlated to the clinical response. Serum HCG levels are rarely elevated in non-trophoblastic tumors, such as lung, breast, pancreas, and bladder cancers.Citation25 Half-life of it in serum is approximately16-24 h, decline of serum levels may be biphasic with a second t½ of 12.8 days.Citation29 The role of chronic kidney disease (CKD) as a cause of elevated HCG levels has not been studied. It is well-known that 30% of HCG being produced is cleared by the kidney and an additional fraction metabolized by it. Retrospective study performed by Soni et al.,Citation52 reported a series of 5 women out of 62 with CKD, who had a positive HCG (≥5 IU/L) test on routine pre-transplant screening at a single transplant center. In this cohort of female CKD patients, HCG levels >5 IU/L were observed in 5/62 (8.1%). The presence of CKD may cause to elevate the further HCG levels in patients. Despite aggressive investigation, their elevated HCG levels remained unexplained. The positive test contributed to delays in transplantation and increased overall cost of treatment. A case report showed persistent elevation of β-HCG levels in a patient with stage 5D chronic kidney disease. Elevated β-HCG could be attributed to a tumor of gestational or non-gestational origin, failed pregnancy, familial high HCG, reduced clearance, and degradation of HCG in CKD or CKD-associated changes of HCG. Immunoassay interference should be considered when patient results and clinical presentation differs. False positive test results may subject patients to unnecessary investigation and it may prevent patients from qualifying for organ transplantation.Citation53
Carcinoembryonic antigen in CKD
Carcinoembryonic antigen (CEA), first described in 1965, was characterized as glycoproteins of 200 kDa. It is excreted by certain embryonic and adult tissues in addition to adenocarcinoma of the digestive organs. Extensive studies of patients bearing primary and metastatic colorectal neoplasms have determined that its primary use is in the detection of local and metastatic cancer recurrence after initial resection of the primary tumor, through periodic post-operative analysis of CEA in serum or plasma. Clinical studies showed that CEA, although originally thought to be specific for digestive tract cancers, may also be elevated in other malignancies and in some non-malignant disorders. CEA testing has significant value in the monitoring of patients with diagnosed malignancies in whom changing concentrations of CEA are observed. A persistent elevation in circulating CEA following treatment is strongly indicative of occult metastatic and/or residual disease. A persistently rising CEA value may be associated with progressive malignant disease and a poor therapeutic response. A declining CEA value is generally indicative of a favorable prognosis and a good response to treatment. Patients who have low pretreatment CEA levels may later show elevations in the CEA level as an indication of progressive disease. Follow-up studies of patients with colorectal, breast, and lung carcinoma suggest that the pre-operative CEA level has prognostic significance. CEA testing is not recommended as a screening procedure to detect cancer in the general population; however, use of the CEA test as an adjunctive test in predicting prognosis and as an aid in the management of cancer patients has been widely accepted.Citation25 Levels of CA 125, Carcino-embryonic antigen (CEA), squamous cell carcinoma antigen (SCC), neuron-specific enolase (NSE) can be falsely elevated and have to be interpreted with caution.Citation30 CEA levels typically return to normal within four to six weeks after successful surgical resection. The major role for CEA levels is in following patients for relapse after intended curative treatment of colorectal cancer. When patients with a normal pre-operative CEA level have cancer recurrence, CEA elevation is a sign in nearly one half of them. The American Society of Clinical Oncology recommends monitoring CEA levels every two to three months for at least two years in patients with stage II or III disease who are surgical candidates.Citation54 Although carcinoembryonic antigen and CA 19-9 have been proposed for use of gastric cancer and squamous cell carcinoma antigen for use in cervical cancer, none of these markers can currently be recommended for routine clinical use.Citation55 The combination of CEA and β2-M determination proved to be most useful in cases of non-small cell lung carcinoma and for follow up of cases. Serial determination of the CEA is recommended with each course of treatment to help in predicting patient’s response and monitoring the disease.Citation56 Carcinoembryonic antigen (CEA) was estimated in plasma from 70 patients with a renal transplant, 105 patients with glomerulonephritis who had received immunosuppressive therapy, and 124 healthy controls. There were raised levels in 30% of those with a renal transplant, 10% of those with glomerulonephritis, and 2% of controls, and levels were higher in current smokers. CEA levels correlated neither with prednisolone dosage nor with number of rejection episodes, after allowing for time after transplantation and smoking habit. Nine of 70 patients with a renal transplant and three of 105 with glomerulonephritis had cancer as skin in seven, cervix uteri in four, and colon in one. CEA was raised in all of four transplant recipients with a visceral cancer (three cervical cancers and one colon cancer), but did not rise in any of the five patients with cutaneous cancer. Raised CEA levels occurring late after a renal allograft should prompt a careful search for visceral cancer.Citation57 Furthermore, serum CEA concentrations strongly tend to duplicate after HD treatment.Citation22 In a study by Bo,Citation58 the diagnostic value of CEA, CA l9-9, NSE, CA 125 in tumors of maintenance hemodialysis patients were decreased, and the detection and diagnostic value of tumor markers of CA 242, CA l53, β-HCG, AFP, human growth hormone (HGH), PSA, fPSA were normal. Additionally, an elevated CEA level in the patients with severe bowel inflammation, such as complicated pseudomembranous colitis (PMC) can be correlated with inflammation.Citation59 The presence of extramedullary involvement in multiple myeloma (MM) patients is a rare clinical manifestation, found in only 4.6% of cases. Talamo et al.,Citation60 reported a case of rectal involvement by MM in which the malignant plasma cells produced serum carcinoembryonic antigen (CEA), a tumor marker more commonly associated with rectal adenocarcinomas.
Chromogranin A in chronic kidney disease
Granins are proteins found in dense core secretory granules of most endocrine and non-endocrine cells as well as central and peripheral neurons. There are two subfamilies: chromogranins, mainly A and B and secretogranins II to VI. Secretogranins I and II are also known as CgB and CgC.Citation61 Chromogranin A belonging to the group of closely related secretory acidic proteins is used as a tumor marker to assess exocytotic sympathoadrenal activity in Pheochromocytoma patients. Its concentrations in plasma are elevated in peptide producing tumors.Citation25 Chromogranin A (CgA) is a 49 kDa acidic hydrophilic protein synthesized in the chromaffin granules of the neuroendocrine cells and is traceable in the blood of healthy subjects at concentrations of less than 30 ng/mL. Elevated levels of CgA in serum are detectable in patients affected by neuroendocrine (NE) or carcinoid tumors, pheochromocytoma, neuroblastoma, small cell lung cancer (SCLC), and prostate cancer.Citation3 Recently, CgA has known as a useful biomarker in hepatocellular carcinoma (HCC) screening, representing a complementary test when the levels of α-FP are not sufficiently diagnostic (<200 ng/mL). Serum CgA determination can also be used to predict the progression or regression of neuroendocrine tumors during the treatment. However, CgA elevation has been rarely reported in patients without cancerous conditions, such as essential hypertension, so that the possibility of false positive results should be considered. CgA is the most ubiquitous and sensitive general marker for the diagnosis of endocrine tumors. In a study by Kurnatowska et al.,Citation62 plasma CgA and blood pressure of 38 chronic hemodialysis patients were measured before and after a mid-week dialysis. Plasma CgA levels were on average 50-fold higher in HD patients than in the controls. In HD patients, plasma CgA corrected for ultrafiltration rates that were significantly increased at the end of dialysis procedure. Therefore, CgA undergoes marked accumulation in renal failure.Citation63 Awadallah et al.,Citation64 revealed that the application of CgA as a tumor marker in the diagnosis of hepatocellular carcinoma (HCC) is to be considered especially in cases with low levels of AFP, as determination of CgA serum values represents a complementary diagnostic tool in monitoring of chronic liver disease patients for detection of HCC. The combined use of both CgA and AFP to detect HCC increases their sensitivity and specificity. Chromogranin A is released with epinephrine and norepinephrine from catecholaminergic cells. Specific endopeptidases cleave chromogranin A into biologically active peptide fragments, including catestatin, which inhibits catecholamine release. Previous studies have suggested that a deficit in this sympathetic “braking” system might be an early event in the pathogenesis of human hypertension. Circulating levels of catestatin were lower among those with hypertensive end-stage renal disease (ESRD) than controls, an unexpected finding given that peptide levels are usually elevated in ESRD because of reduced renal elimination. So, common variants in chromogranin A associate with the risk of hypertensive ESRD in blacks.Citation65 High serum levels of CgA have also been demonstrated in patients with ovarian cancer. The abnormal productions of CgA suggest that this circulating glycoprotein can indeed affect several aspects of the complex interplay between tumor-associated vessels and neoplastic cells. According to previous studies, Catania et al.,Citation66 found that CgA depicted a significant trend in association with high-grade disease. Wise-Draper et al.Citation67 reported a case of 78-year-old male with a high-grade post-transplant lymphoproliferative disorder (PTLD) and metastatic pancreatic neuroendocrine tumor (PNET) about 14 years after kidney transplantation. To evaluate oxidative stress and CgA levels in uremia and dialysis, study showed that uremia is the major source of the increase in oxidative stress and CgA levels in patients with end-stage renal disease.Citation68
Other tumor markers
C-reactive protein (CRP) is an acute phase protein synthesized by hepatocytes in the liver in response to interleukin-6 (IL-6) cytokine induction according to inflammatory process as a result of a host immune response. The present results suggest that CRP levels are a more consistent indicator of cancer risk than some tumor markers. Furthermore, the association between cancer incidence and CRP as an inflammatory marker may be tumor type specific and increased level of CRP may show a stronger association with risk of cancer incidence, recurrence, and death.Citation69 Trefoil factor family (TFF) members are small secreted proteins that are co-expressed with mucins by the epithelial cells lining the gastrointestinal tract. Serum and urine TFF3 levels were significantly higher in the patients with gastric and colorectal cancer compared with the healthy individuals. Data demonstrated that serum TFF3 can be applied as an effective biomarker for the detection of tumor stages, distant metastasis, and pharmacodynamic marker of responses to chemotherapy in gastrointestinal cancer. Urine TFF3 is a different indicator than serum levels and could also be a biomarker for the early detection of renal dysfunction.Citation70 Elevated levels of serum gastrin (SG) have been associated with tumorigenic effects in a number of gastrointestinal cancers. A high SG level (>80 pg/mL), intestinal metaplasia, and a pepsinogen I/II ratio <3 were independently associated with an increased risk of epithelial lesions.Citation71 Cytokeratin fragment 21-1 is an antigenic determinant present on 40 KD protein of the cytokeratin 19. This antigen is expressed in normal, simple epithelium as well as in proliferating epithelium. Cyfra 21-1 is used as a tumor marker for non-small cell lung cancer (NSCLC), such as squamous cell carcinoma (SCC), adenocarcinoma and large cell carcinomas. This tumor marker shows highest sensitivity for SCC in lung. Both Cyfra 21-1 and CA 19-9 have improved the sensitivity for the detection of adenocarcinoma of lung.Citation25 CA 549 is a high molecular weight circulating glycoprotein antigen associated with breast cancer. Elevated levels of CA 549 are observed in serum of advanced breast cancer by immunoassay. However, use of CA 19-5 and CA 50 together improves sensitivity in detecting pancreatic and other carcinomas.
Tissue polypeptide antigen (TPA), which is regarded as a marker of cell proliferation, is a mixture of proteolytic fragments containing the relatively stable α-helical rod domains of simple epithelium-type cytokeratin. The marked elevation of serum TPA is reported in variety of cancers, such as breast, lung, gastrointestinal, urological, gynecological cancer. TPA is known to be a sensitive but non-specific tumor marker. However, TPA along with CEA uses as aid for monitoring of lung, bladder, breast, colorectal, and ovarian carcinomas. TPA is also reported to differentiate between cholangiocarcinoma and hepatocarcinomas. Calcitonin, a low molecular weight circulating peptide hormone, that synthesized by C cells of the thyroid, is used as tumor marker and its increased concentration is reported in malignancies with skeletal metastases. Serum calcitonin concentrations are also reported to increase in medullary carcinoma of the thyroid, bronchogenic carcinoma, small cell lung cancer, breast, liver, lung, renal cancers, and carcinoid tumors. Catecholamines, Cathepsin D, epidermal growth factor receptor (EGFR), estrogen receptor (ER), progesterone receptor (PR), ferritin, homovanillic acid (HVA) and vanillylmandelic acid (VMA), hydroxyindoleacetic acid (5-HIAA), interleukin 2 receptor/tac antigen (IL-2R), lipid-associated sialic acid in plasma (LASA-P), neuron-specific enolase (NSE), oncogene P21 RAS, tumor suppressor gene P53, BRCA 1 and BRCA 2, parathyroid hormone-related peptide (PTH-RP), PS2, squamous cell carcinoma (SCC) antigen, monoclonal immunoglobulin/paraprolactin, free soluble CD52 have known as tumor markers.Citation25 Higher serum vascular adhesion protein (VAP-1) and CKD can independently predict future development of cancers in type II diabetic subjects.Citation72 Common serum tumor markers have considered for different clinical use (). In addition, some studies have evaluated association of these tumor markers with trace elements. Turkmen et al.Citation73 in the correlation analysis showed a positive correlation between serum zinc (Zn) levels and CA 15-3 and a negative correlation between serum Zn levels and AFP in hemodialysis patients. Repletion of the zinc may be beneficial to prevent various cancers in this population. Data suggest that grouping cancer patients according to renal function, especially estimated glomerular filtration rate (eGFR), may be one way to determine specific risk groups.
Table 2. Clinical use of tumor markers in screening, diagnosis, follow-up, and monitoring of cancer patients.
Combination of serum tumor markers
Grouping tumor markers, such as HE4 and CA 125 are valuable in ovarian cancer screening, diagnosis, and prognosis. The measurement of some of the tumor markers including alpha-fetoprotein and β-HCG may be beneficial in hemodialysis patients ().
Table 3. Panel of complementary serum tumor markers in detection of cancer.
Conclusions
Each tumor marker has a variable profile of usefulness for screening, determining diagnosis and prognosis, assessing response to therapy, and monitoring for cancer recurrence. However, none of the tumor markers reported to date has all characteristics of an ideal tumor marker. Therefore, with high suspicion to malignancy in chronic kidney disease patients and renal allograft recipients, these tumor markers can be helpful in clinical diagnosis of certain tumors and follow-up of cancer patients. Hence, it seems measurement of prostate-specific antigen, alpha-fetoprotein, and β-human chorionic gonadotropin can be reliable in dialysis patients and total prostate-specific antigen and β2-microglobulin are valuable in post-renal transplant recipients.
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Weng P, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer-specific mortality in chronic kidney disease: Longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6(5):1121–1128.
- Estakhri R, Ghahramanzade A, Vahedi A, Nourazarian A. Serum levels of CA15-3, AFP, CA19-9 and CEA tumor markers in cancer care and treatment of patients with impaired renal function on hemodialysis. Asian Pacific J Cancer Prev. 2013;14(3):1597–1599.
- Coppolino G, Bolignano D, Rivoli L, Mazza G, Presta P, Fuiano G. Tumor markers and kidney function: A systematic review. Biomed Res Int. 2014;2014:1–9.
- Hussain S, Abbas G. Prostate specific antigen levels in pre-dialysis chronic kidney disease patients. Saudi J Kidney Dis Transpl. 2010;21(2):354–356.
- Bruun L, Savage C, Cronin AM, Hugosson J, Lilja H, Christensson A. Increase in percent free prostate-specific antigen in men with chronic kidney disease. Nephrol Dial Transplant. 2009;24(4):1238–1241.
- Joseph DA, Thompson T, Saraiya M, Werny DM. Association between glomerular filtration rate, free, total, and percent free prostate-specific antigen. Urology. 2010;76(5):1042–1046.
- Mercadal L. Tumor markers in chronic kidney disease. Nephrol Ther. 2015;11(2):122–124.
- Arik N, Adam B, Akpolat T, Has Kil, S, Tabak S. Serum tumour markers in renal failure. Int Urol Nephrol. 1996;28(4):601–604.
- García-Sánchez C, Corchuelo-Maillo C, Congregado-Ruiz CB, et al. PSA levels in patients on hemodialysis treatment. Arch Esp Urol. 2013;66(10):939–944.
- Bruun L, BJork T, Hans L, Becker C, Gustafsson O, Christensson A. Percent-free prostate specific antigen is elevated in men on hemodialysis or peritoneal dialysis treatment. Nephrol Dial Transplant. 2003;18(3):598–603.
- Sumura M, Yokogi H, Beppu M, Honda H. Diagnostic value of serum prostate-specific antigen in hemodialysis patients. Int J Urol. 2003;10(5):247–250.
- Khairullah QT, Pamatmat SD, Chatha M, Provenzano R, Telang D, Temple M. Early detection of prostate cancer in the ESRD population. Clin Nephrol. 2004;61(5):308–315.
- Wada Y, Nakanishi J, Takahashi W, et al. Mass screening for prostate cancer in patients with end-stage renal disease: A comparative study. BJU Intl. 2006;98(4):794–797.
- Tarhan F, Orcun A, Kucukercan I, Camursoy N, Kuyumcuoglu U. Effect of hemodialysis on serum complexed prostate-specific antigen levels. Scand J Urol. 2007;41(5):382–386.
- Holley JL. Screening, diagnosis and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol. 2007;2(3):604–610.
- Kleinclauss F, Gigante M, Neuzillet Y, et al. Prostate cancer in renal transplant recipients. Nephrol Dial Transplant. 2008;23(7):2374–2380.
- Cormier L, Lechevallier E, Barrou B, et al. Diagnosis and treatment of prostate cancers in renal transplant recipients. Transplantation. 2003;75(2):237–239.
- Mahdavi R, Zeraati A, Tavakkoli M, Aghamohammadpour K, Ghoreifi A. Effect of kidney transplantation on early and late post-transplant prostate-specific antigen levels. Saudi J Kidney Dis Transpl. 2014;25(2):362–366.
- Chamie K, Ghosh PM, Koppie TM, Romero V, Troppmann C, de Vere White RW. The effect of sirolimus on prostate-specific antigen (PSA) levels in male renal transplant recipients without prostate cancer. Am J Transplant. 2008;8(12):2668–2673.
- Lucarelli G, Ditonno P, Bettocchi C, et al. Diagnostic and prognostic role of preoperative circulating CA 15-3, CA 125 and Beta-2 microglobulin in renal cell carcinoma. Dis Markers. 2014;2014:1–9.
- Engin H, Borazan A, Aydemir S, Yilmaz A. Assessment of tumor markers in patients with chronic renal failure. Turkish J Cancer. 2007;37(4):143–147.
- Tzitzikos G, Saridi M, Filippopoulou T, et al. Measurement of tumor markers in chronic hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(1):50–53.
- Xiaofang Y, Yue Z, Xialian X, Zhibin Y. Serum tumor markers in patients with chronic kidney disease. Scand J Clin Lab Invest. 2007;67(6):661–667.
- Kanaan N, Goffin E, Pirson Y, Devuyst O, Hassoun Z. Carbohydrate antigen 19-9 as a diagnostic marker for hepatic cyst infection in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2010;55(5):916–922.
- Malati T. Tumor markers: An overview. Indian J Clin Biochem. 2007;22(2):17–31.
- Zeferos N, Digenis GE, Christophoraki M, et al. Tumor markers in patients undergoing hemodialysis or kidney transplantation. Nephron. 1991;59(4):618–620.
- Oberbauer R, Banyai S, Schmidt A, Kornek G, Scheithauer W, Mayer G. Serum markers after renal transplantation. Transplantation. 1996;62(10):1506–1509.
- Wood WG, Steinhoff J, Kessler AC. Anomalous tumor marker concentrations in renal transplant patients. Eur J Clin Chem Clin Biochem. 1993;31(2):75–82.
- Duffy MJ, McGing P. Guidelines for the Use of Tumor Markers. 3rd ed., Ireland: Scientific Committee of the Association of Clinical Biochemists (ACBI); 2005.
- Rao SN. Cancer screening in end-stage renal disease. Saudi J Kidney Dis Transpl. 2009;20(5):737–740.
- Nagy B Jr, Krasznai ZT, Balla H, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012;49(4):377–380.
- Hamed EO, Ahmed H, Sedeek OB, Mohammed AM, Abd-Alla AA, Abdel Ghaffar HM. Significance of HE4 estimation in comparison with CA 125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol. 2013;8:11.
- Simmons AR, Baggerly K, Bast Jr RC. The emerging role of HE4 in the evaluation of advanced epithelial ovarian and endometrial carcinomas. Oncology (Williston Park). 2013;27(6):548–556.
- Anastasi E, Granato T, Falzarano R, et al. The use of HE4, CA 125 and CA 72-4 biomarkers or differential diagnosis between ovarian endometrioma and epithelial ovarian cancer. J Ovarian Res. 2013;6(44):1–8.
- Yontem M. Investigation of the serum levels of tumor markers in the patients chronic renal failure. Int J Sci Technol Res. 2015;1(2):115–119.
- Dieplinger H, Ankerst DP, Burges A, et al. Afamin and apolipoprotein A-IV: Novel protein markers for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1127–1133.
- Dieplinger H, Dieplinger B. Afamin-A pleiotropic glycoprotein involved in various disease states. Clin Chim Acta. 2015;446:105–110.
- Freue GV, Sasaki M, Meredith A, et al. Proteomic signatures in plasma during early acute renal allograft rejection. Mol Cell Proteomics. 2010;9(9):1954–1967.
- Myata T, Jadoul M, Kurokawa K, Van Yepersele De Strihou C. β2-microglobulin in renal disease. J Am Soc Nephrol. 1998;9:1723–1735.
- Li K, Du H, Lian X, et al. Characterization of β2-microglobulin expression in different types of breast cancer. BMC Cancer. 2014;14:750.
- Nomura T, Huang WC, Zhau HE, Josson S, Mimata H, Kaur M. β2-Microglobulin-mediated signaling as a target for cancer therapy. Anticancer Agents Med Chem. 2014;14(3):343–352.
- Chmielewski M, Cohen G, Wiecek A, Jesus Carrero J. The peptidic middle molecules: Is molecular weight doing the trick? Semin Nephrol. 2014;34(2):118–134.
- Jiang Q, Patima S, Ye DX, Pan HY, Zhang P, Zhang ZY. Upregulation of β2-microglobulin expression in progressive human oral squamous cell carcinoma. Oncol Rep. 2012;27(4):1058–1064.
- Blum C, Graham A, Yousefzadeh M, et al. The expression ratio of Map7/B2M is prognostic for survival in patients with stage II colon cancer. Int J Oncol. 2008;33(3):579–584.
- Gross M, Top I, Laux I, et al. β-2-Microglobulin is an androgen-regulated secreted protein elevated in serum of patients with advanced prostate cancer. Clin Cancer Res. 2007;13(7):1979–1986.
- Rodriguez J, Cortes J, Talpaz M, et al. Serum β-2 microglobulin levels are a significant prognostic factor in Philadelphia chromosome-positive chronic myelogenous leukemia. Clin Cancer Res. 2000;6:147–152.
- Dajak M, Ignjatovi S, Biljana Stojimirovi B, Gaji S, Majki-Singh N. Beta-trace protein as a marker of renal dysfunction in patients with chronic kidney disease: comparison with other renal markers. J Med Biochem. 2010;29(2):66–72.
- Astor BC, Muth B, Kaufman DB, Pirsch JD, Hofmann RM, Djamali A. Serum β2-microglobulin at discharge predicts mortality and graft loss following kidney transplantation. Kidney Int. 2013;84:810–817.
- Zumrutdal A. Role of β2-microglobulin in uremic patients may be greater than originally suspected. World J Nephrol. 2015;4(1):98–104.
- Raikou VD, Kyriaki D. The relationship between glycemic control, beta2-microglobulin and inflammation in patients on maintenance dialysis treatment. J Diabetes Metab Disord. 2015;14(34):1–6.
- Huang WC, Havel JJ, Zhau HE, et al. β2-Microglobulin signaling blockade inhibited androgen receptor axis and caused apoptosis in human prostate cancer cells. Clin Cancer Res. 2008;14(17):5341–5347.
- Soni S, Menon MC, Bhaskaran M, Jhaveri KD, Molmenti E, Muoio V. Elevated human chorionic gonadotropin levels in patients with chronic kidney disease: Case series and review of literature. Indian J Nephrol. 2013;23(6):424–427.
- Hayden Y, Bezuidenhout E, de Lange W, Van Schalkwyk O. Persistent elevation of BHCG-levels in a patient with stage 5D chronic kidney disease. J Endocrinol Metab Diabetes South Africa. 2015;20(1):38–46.
- Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician. 2003;68(6):1075–1082.
- Sturgeon CM, Duffy MJ, Hofmann BR, et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56(6):e1–e48.
- Ragab H, Abd El Maksoud N, Essam T. The impact of use serum carcinoembryonic antigen and Beta2-microglobulin in monitoring bronchogenic carcinoma therapy. J Genet Eng Biotechnol. 2007;5(1-2):43–50.
- Myers JB, Frost M, Coates AS, Mathews JD, Kincaid-Smith P. Carcinoembryonic antigen in renal allograft recipients and immunosuppressed renal patient. Aust N Z J Med. 1977;7(1):16–19.
- Bo L. Clinical value of serum tumor markers detection in maintenance hemodialysis patients. Chin J Clin Oncol Rehabil. 2013;3:201–203.
- Nam DI, Kang C, Jung IH, Moon HG, Youn BR, Lee NH. Pseudomembranous colitis: A complicated case with transient increase of carcinoembryonic antigen. Ewha Med J. 2015;38(1):54–58.
- Talamo G, Barochia A, Zangari M, Loughran TP. Multiple myeloma presenting as CEA-producing rectal cancer. Rare Tumors. 2010;2(1):e4.
- d’Herbomez M, Do Cao C, Vezzosi D, Borzon-chasot F, Boudin E. Chromogranin A assay in clinical practice. Ann Endocrinol. 2010;71(4):274–280.
- Kurnatowska I, Nowicki M. Serum chromogranin A concentration and intradialytic hypotension in chronic hemodialysis patients. Int Urol Nephrol. 2006;38(3-4):701–705.
- Tramonti G, Ferdeghini M, Annichiarico C, et al. Relationship between renal function and blood level of chromogranin A. Ren Fail. 2001;23(3-4):449–457.
- Awadallah AM, Issa HA, Soliman MS. Evaluation of serum chromogranin A as a useful tumor marker for diagnosis of hepatocellular carcinoma. J Am Sci. 2011;7(1):999–1007.
- Salem RM, Cadman PE, Chen Y, et al. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008;19(3):600–614.
- Catania VE, Vinci E, Madeddu R, et al. Chromogranin A serum levels in elderly patients with ovarian cancer. Acta Med Mediterranea. 2014;30:1053–1057.
- Wise-Draper T, Qualtieri J, Mogilishetty G, Latif T. Concurrent presentation of high-grade lymphoma and metastatic pancreatic neuroendocrine tumor 14 years after renal transplant. Hematol. 2014;3(4):112–115.
- Castoldi G, Antolini L, Bombardi C, et al. Oxidative stress biomarkers and chromogranin A in uremic patients: Effects of dialytic treatment. Clin Biochem. 2010;43(18):1387–1392.
- Elshabrawy S, Farid A, El-Beddini M, Osman A, El-Deeb S. Evaluation of C-reactive protein as a probable factor for cancer diagnosis. Life Sci J. 2012;9(4):2796–2803.
- Xiao L, Liu YP, Xiao CX, Ren JL, Guleng B. Serum TFF3 may be a pharmacodynamic marker of responses to chemotherapy in gastrointestinal cancers. BMC Clin Pathol. 2014;14:26.
- Kim BC, Jung SW, Kim JB, et al. Serum gastrin levels in different stages of distal gastric carcinogenesis: Is there a role for serum gastrin in tumor growth? Turk J Gastrointerol. 2014;25(6):611–618.
- Yu TY, Li HY, Jiang YD, et al. Serum vascular adhesion protein-1 level predicts risk of incident cancers in subjects with type II diabetes. Cancer Epidemiol Biomarkers Prev. 2014;23(7);1366–1373.
- Turkmen K, Ecder T, Turk S. The relationship between serum zinc levels and tumor markers in hemodialysis patients. Eur J Gen Med. 2014;11(3):174–178.
