Abstract
Podocyte injury is a vital factor, which induces massive proteinuria. Studies have shown that tacrolimus (TAC) protected podocyte via stabilizing cytoskeleton. Our latest study indicates that calcineurin binding protein 1 (Cabin1) undergoes nuclear translocation during podocytes injury. Whether TAC targets on Cabin1 during podocyte injury is still not clear. This study establishes non-immunological proteinuric model. To observe the effect of the treatment of TAC on Cabin1 expression in 5/6 nephrectomized rats. Sprague–Dawley rats were injected with TAC (0.2 mg/kg/day) for 4–8 weeks after 5/6 nephrectomy. Then, rats were sacrificed in the eighth week after operation, renal tissues were processed for morphological studies under light and electrical microscope. Cabin1 expression and distribution were detected by western blot and indirect immunofluorescence staining. In 5/6 nephrectomized rats, urinary protein excretion reached 90.2 ± 30.1 mg/24 h, glomerular sclerosis index and tubulointerstitial fibrosis score were significantly increased, and widespread of podocyte foot processes fusion was found. Moreover, Cabin1 protein expression was markedly increased, and its distribution became much more obviously in podocytes nuclei. In TAC treated rats, urinary protein excretion significantly decreased (44.9 ± 22.5 mg/24 h), glomerular sclerosis and tubulointerstitial fibrosis were alleviated, and podocyte foot processes fusion was inhibited. Furthermore, TAC alleviated the increased protein expression and abnormal distribution of Cabin1. In conclusion, TAC restores podocyte injury and stabilizes the expression of Cabin1. Cabin1 may become a new target to demonstrate the mechanism of TAC in podocyte injury.
Key words:
Introduction
Proteinuria is a critical independent risk factor for the progression of primary and secondary renal diseases.Citation1 As the third layer of glomerular filtration barrier (GBM), podocyte depletion leads to the dysfunction of GBM, which is closely related to proteinuria.Citation2 It has been proved that if massive proteinuria effectively being alleviated by steroids and/or immunosuppressive agents, the prognosis of patients will be better.Citation3–5 To our disappointment, there are partly patients showing resistance to steroids and/or immuno-suppressive agents. In that case, it is necessary to explore effective and safe strategy for those patients.
Tacrolimus (TAC), as a calcineurin (CN) specific inhibitor, is mainly used for patients who receive organ transplants. Studies have indicated that TAC effectively decreased massive proteinuria in various glomerular diseases, especially for those patients who suffer from refractory nephrotic syndrome.Citation6,Citation7 Faul et al. demonstrated that ciclosporin A (CsA), another CN inhibitor, reduced proteinuria and protected kidney function by stabilizing podocyte actin cytoskeleton.Citation8 Latest study showed that TAC targets on angiopoietin-like-4 (Angptl4) and predict earlier podocyte injury in minimal change disease.Citation9 However, TAC treatment may also induce some serious side effects, such as hypertension, pathoglycemia and the damage of interstitial.Citation10,Citation11 In order to effectively and safely decrease proteinuria, further studies need to be done to illuminate the exactly mechanism of TAC in podocyte injury.
Calcineurin binding protein 1 (Cabin1) is an endogenous inhibitor of CN, it interacts with CN via its C terminus.Citation12 Moreover, Cabin1 interacts with p53 via its N terminus. Our latest study found that Cabin1 localized in glomerular podocyte and undergone nuclear translocation during podocyte injury,Citation13 while knockdown of Cabin1 with siRNA induced lower expression of p53 in cultured mouse podocyte cell line.Citation14
In order to investigate the mechanism of TAC in proteinuria and podocyte injury, we established 5/6 nephrectomized rats as a model, to observe the relationship of TAC treatment and the expression of Cabin1 in 5/6 nephrectomized rats.
Materials and methods
Establishment of 5/6 nephrectomized rats and treated with TAC
With the approval of the Animal Ethics Committee (Guangdong Province), experiments were conducted on Sprague–Dawley rats (Medical Laboratory Animal Center of Guangdong Province, Guangzhou, China). In this study, we strictly followed the principles of laboratory animal care. The experiments were performed with male Sprague–Dawley rats weighing 200–250 g. Each 5/6 nephrectomy group and TAC treatment group included five rats and the control group included six. 5/6 Nephrectomy was performed under general anesthesia. By retroperitoneal approach, two poles of the left kidney were removed by polectomy. One week later, the right kidney was removed. Sham-operated rats underwent anesthesia, ventral laparotomy and manipulation of the renal pedicles without removal of renal mass. TAC treatment rats were given TAC at a dosage of 0.2 mg/kg/ day by intragastric administration from 4 to 8 weeks after 5/6 nephrectomy (TAC was obtained from the pharmacy at the second affiliated hospital of Guangzhou Medical University, Guangzhou, China). Blood and urine samples were collected at 4 and 8 weeks after the operation. Blood samples were drawn from the retro-orbital sinus, while 24 h urine samples were collected in metabolism cages. Serum creatinine, blood urea nitrogen (BUN) levels and 24 h urinary protein excretion were determined by colorimeter. Rats were sacrificed at 8 weeks after operation then remnant or control kidneys were removed. A third of kidney tissues was stored in −80 °C in the refrigerator for western blot and then fixed in 4% paraformaldehyde, dehydrated in graded alcohol and embedded in paraffin.
Morphological studies by light microscopy
Kidney tissues were fixed in 10% formalin, dehydrated in graded alcohol and embedded in paraffin. Two-micrometer sections were cut and stained with H&E, as well as Masson’s trichrome. All slides were evaluated by the experienced pathologist at microscopy (Olympus CH20BIMF200, Tokyo, Japan). The mean score per glomerulus in each kidney was determined as the sclerosis index. Similarly, sections from each kidney cortex were graded for the presence of interstitial fibrosis. The scale for each rat was reported as the mean of 20 random high-power (×400) fields per section.
Morphological studies by transmission electron microscopy
Renal cortex samples were processed through a primary and a secondary fixation, acetone dehydration, and then EponSpurr’s resin infiltration. Samples were rocked overnight and then embedded and polymerized at 60 °C for 24 h. Thin sections were collected and stained with uranyl acetate and lead citrate. A transmission electron microscopy (Philips CM10; Eindhoven, The Netherlands) was used to observe the samples.
Western blotting
Renal cortex samples were collected and lysed in lysis buffer (Cell Signaling Technology, Danvers, MA). Equal amounts protein loadings were separated by SDS–PAGE on 8% gels and electrophoretically transferred to a nitrocellulose membrane. Non-specific binding sites were blocked with 5% non-fat milk powder in PBS for 1 h. Membranes were incubated in primary antibody (Cabin1, Novus, Littleton, CO; GAPDH, Kangchen, Shanghai, China) overnight at 4 °C. Finally, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. An enhanced chemiluminescence reagent (Santa Cruz Biotechnology) was used to detect the bound antibodies. The specific protein bands were scanned and qualified by the use of a densitometer (Alpha Fluorchem TM 8900, AlphaInnotech, Witec, Littau, Switzerland)
Immunofluorescence staining
The sections of paraffin-embedded renal tissues were rehydrated and subjected to antigen repairing pot for antigen retrieval overnight. Then renal sections were permeabilized with 0.3% Triton-100 for 5 min, followed by being blocked with 5% bovine serum albumin for 60 min. To observe the distribution of Cabin1, the sections were incubated with primary antibody (Cabin1, Novus) overnight at 4 °C, followed by incubation with goat anti-rabbit IgG (Cell Signaling Technology, Boston, MA) for 1 h. Finally, the renal sections were mounted on glass slides with Mounting Medium (R&D Systems, Minneapolis, MN) and viewed by using confocal fluorescence microscope (Zeiss LSM510, Oberkochen, Germany). All exposure settings were kept constant for each section. Mean fluorescence intensity of Cabin1 in glomeruli was analyzed by ImageJ (NIH, Bethesda, MD).
Statistical analysis
In our study, all experiments were carried out in triplicate. For comparison of continuous variables between two groups, statistical significance was assessed by a t test. For comparisons between more than two groups, analysis was performed using one-way ANOVA (analysis of variance). Pairwise comparisons were evaluated by the Student–Newman–Keuls procedure or Dunnett’s T3 procedure when the assumption of equal variances did not hold. p < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS software (SPSS, Inc., Chicago, IL).
Results
TAC reduced proteinuria in 5/6 nephrectomized rats
Urinary protein excretion gradually increased up to 90.2 ± 30.1 mg/24 h at 8 weeks after 5/6 nephrectomy. While serum creatinine and BUN level in 5/6 nephrectomized rats were significantly higher than sham-operated rats, compared with the model group, serum creatinine and BUN levels inclined to decrease at 8 weeks after operation in TAC treated group. A statistically significant reduction of proteinuria (44.9 ± 22.5 mg/24 h) was observed in the TAC treated group ().
Table 1. Urinary protein excretion and biochemical parameters of blood measurement of rats.
TAC delayed glomerular sclerosis and tubular fibrosis in 5/6 nephrectomized rats
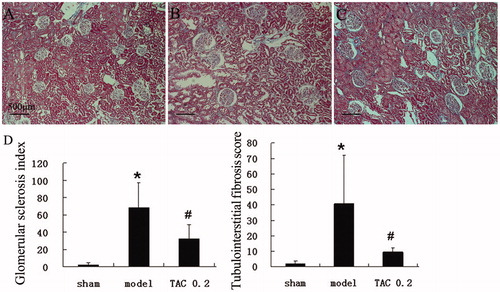
At 8 weeks after 5/6 nephrectomy, glomeruli showed obviously mesangial expansion and glomerular sclerosis, as well as interstitial fibrosis and tubular atrophy. After being treated by TAC from 4 to 8 weeks after the operation, rats were sacrificed at the eighth week after nephrectomy. In TAC treated rats, the range of glomerular sclerosis and interstitial fibrosis was significantly less than the model group, tubular atrophy was also alleviated. Both glomerular sclerosis index and tubulointerstitial fibrosis score in TAC treated rats were significantly lower than nephrectomized rats (.
Figure 1. TAC delayed glomerular sclerosis and tubular fibrosis in 5/6 nephrectomized rats. Then, renal sections were detected by light microscopy after Masson staining (original magnification: ×100). (A) Normal renal cortex. (B) In 5/6 nephrectomized rats, glomeruli showed obviously mesangial expansion and glomerular sclerosis, as well as interstitial fibrosis and tubular atrophy. (C) In TAC treated rats, the range of glomerular sclerosis and interstitial fibrosis was significantly less than the model group, tubular atrophy was also alleviated. (D) Glomerular sclerosis index and tubulointerstitial fibrosis score were significantly increased in the model group, which were obviously decreased by TAC. *p < 0.05 versus sham-operated rats, #p < 0.05 versus 5/6 nephrectomized rats.
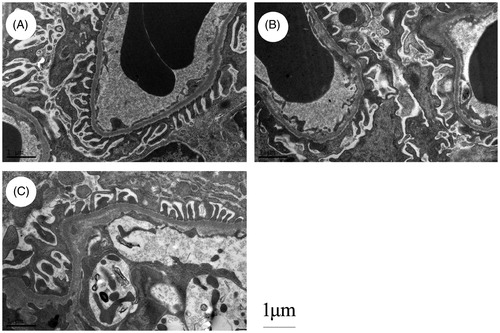
TAC restored podocyte process damage in 5/6 nephrectomized rats
Sham-operated rats showed a “zipper-like” appearance, foot processes from the cell body of podocytes regularly attached to the GBM. Widespread of podocyte foot processes fusion was found in rats at 8 weeks after 5/6 nephrectomy. Treated by TAC significantly prevented the fusion of foot process and preserved the “zipper-like” appearance (.
Figure 2. TAC restored podocyte process damage in 5/6 nephrectomized rats (original magnification: ×13,500). (A) Sham-operated rats showed a “zipper-like” appearance, foot processes from the cell body of podocytes regularly attached to the GBM. (B) Widespread of podocyte foot processes fusion was found in rats at 8 weeks after 5/6 nephrectomy. (C) TAC significantly prevented the fusion of podocyte foot process and maintained the “zipper-like” appearance.
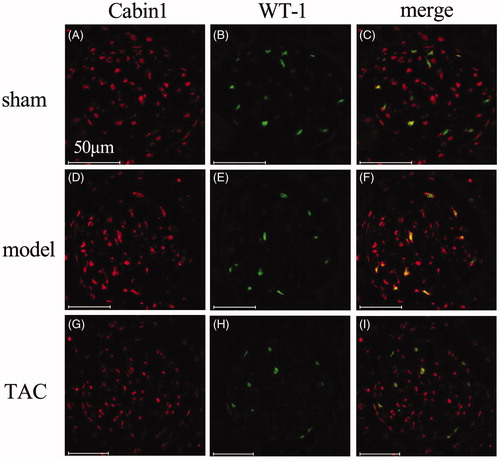
TAC stabilized the distribution of Cabin1 in 5/6 nephrectomized rats
WT-1 is a podocyte nuclear specific marker. Cabin1 partially colocalized with WT-1 in podocytes nuclei in sham-operated rats. While in the remnant kidney of 5/6 nephrectomized rats, the overlap of Cabin1 and WT-1 in podocyte nuclei significantly increased. After treated by TAC for 4 weeks, the distribution of Cabin1 in podocyte nuclei maintained to the original pattern of sham-operated rats (.
Figure 3. TAC stabilized the distribution of Cabin1 in 5/6 nephrectomized rats (original magnification: ×400). (A–C) In sham-operated rats, Cabin1 was mainly localized in glomerular innate cells and partly overlap with WT-1 in the nuclei of podocytes. (D and E) In 5/6 nephrectomized rats, the overlap of Cabin1 and WT-1 in podocyte nuclei was much more obvious than sham-operated rats. (G–I) In TAC treated rats, the distribution of Cabin1 in podocyte nuclei maintained to the original pattern of sham-operated rats.
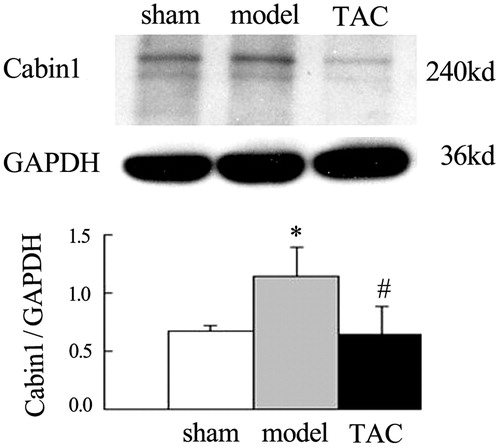
TAC decreased the elevation of Cabin1 protein expression in the renal cortex of nephrectomized rats
Western blot analysis showed relatively low level protein expression of Cabin1 in sham-operated rats. However, Cabin1 protein levels were obviously increased at 8 weeks after operation in 5/6 nephrectomized rats. While compared with the model group, treated by TAC for 4 weeks significantly decreased the elevation protein expression of Cabin1 (.
Figure 4. TAC decreased the elevation of Cabin1 protein expression in the renal cortex of nephrectomized rats. The abundance of Cabin1 in renal cortex was determined by western blot. Western blot analysis showed that relatively low level protein expression of Cabin1 in sham-operated rats. However, Cabin1 protein levels were obviously increased at 8 weeks after operation in 5/6 nephrectomized rats. While compared with the model group, treated by TAC for 4 weeks significantly decreased the elevation protein expression of Cabin1. *p < 0.05 versus sham-operated rats, #p < 0.05 versus 5/6 nephrectomized rats.
Discussion
Proteinuria is regarded as one of the most critical predictive factors of long-term prognosis for patients who suffer from kidney diseases.Citation15 Steroids and/or immunosuppressive agents are the most common agents to reduce proteinuria in kidney diseases. Yet some patients still present with massive proteinuria after receiving above treatment. How to establish an effective strategy to treat patients with refractory proteinuria has not been solved up till now.
TAC, as one of the specific CN inhibitors, is effective to induce massive proteinuria remission in many proteinuric kidney diseases. Zhang et al. found that refractory IgA nephropathy (IgAN) patients who treated with TAC induced rapid proteinuria remission.Citation16 Li et al. reported that TAC significantly reduced proteinuria in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR.Citation17 Roberti and Vyas indicated that TAC was useful for improving proteinuria remission in children with steroid-resistant nephrotic syndrome.Citation18 Our study showed that urinary protein excretion reached 90.2 ± 30.1 mg/24 h in rats at 8 weeks after 5/6 nephrectomy. Treated by TAC from 4 to 8 weeks significantly decreased proteinuria in nephrectomized rats, although high dose and long-term treatment of TAC may induce tubular fibrosis and the damage of kidney function.Citation19 Compared with the model group, treated by low dose of TAC (0.2 mg/kg/day) for 4 weeks, both glomerular sclerosis index and tubular fibrosis score significantly decreased.
5/6 Nephrectomized rats is a non-immunological proteinuric model. Our results indicated that TAC alleviated proteinuria via restoring podocyte process damage. Many studies have suggested that TAC or CsA directly targeted on podocyte specific molecule or cytoskeleton. Faul et al. reported that podocyte cytoskeleton disruption which induced by CN could be alleviated by CsA.Citation8 Zhang et al. also indicated that TAC might improve proteinuria remission of IgAN patients through podocyte cytoskeleton stabilization.Citation16 Recently, researchers found TAC protected podocytes from injury in lupus nephritis patients and lupus nephritis mice model by restoring cytoskeleton disruption and inhibiting podocyte apoptosis.Citation20 Li et al. demonstrated that both serum and urinary of Angptl4 rapidly up-regulated prior to heavy proteinuria in minimal change disease patients, while TAC significantly promoted podocyte repair and reduced Angptl4 expression.Citation9 Researchers suggested that TAC inhibited podocyte apoptosis through lowering TRPC6 expression.Citation21
Cabin1 is a natural selective inhibitor of CN, targeted inhibition of CN by Cabin1 prevented cardiomyocyte hypertrophyCitation22 and regulated the synaptic endocytosis of neurotransmitter vesicles.Citation23 Furthermore, researchers demonstrated that overexpression of Cabin1 in rheumatoid arthritis mice induced fibroblast-like synoviocytes apoptosis and alleviated arthritis.Citation24 Our results showed that Cabin1 protein expression significantly increased in nephrectomized rats, accompanied with obviously podocyte damage, while TAC restored podocyte damage and stabled the expression of Cabin1. As researchers found CN activation promoted apoptosis of glomerular podocytes both in vitro and in vivo, which could be blocked by TAC.Citation25 The overexpression of Cabin1 in the remnant kidney of nephrectomized rats might be a compensatory mechanism to inhibit CN activity. And Cabin1 expression returned to normal level after CN was being inhibited by TAC. The relationship of Cabin1 and CN in podocyte injury needs to be illuminated by further study.
This study also indicated that the distribution of Cabin1 obviously increased in podocyte nuclei in 5/6 nephrectomized rats, while TAC maintained the original pattern of Cabin1. It has been indicated that Cabin1 played an important role in signal transduction pathway. Our previous studies showed that Cabin1 localized in glomerular podocyte and undergone nuclear translocation during podocyte injury.Citation13 Researchers found Cabin1 exported from nucleus via calmodulin-dependent protein kinase IV in the process of T cell activation.Citation26 Jang et al. suggested that Cabin1 interacted with p53 via its N terminus and regulated p53 in tumor cells.Citation27 Whether Cabin1 translocated to nucleus and regulated p53 during podocyte injury? Our latest published article showed that knockdown of Cabin1 with siRNA induced lower expression of p53 in cultured mouse podocyte cell line.Citation14 On the contrary, researchers found Cabin1 retarded lymphoma cells apoptosis by negatively regulating p53.Citation28 The function of Cabin1 in podocyte may be completely different from cancer cell. Interestingly, we found TAC restored the abnormal distribution of Cabin1 in podocyte. CN activation induced nuclear factor of activated T cells (NFAT) translocated to nucleus and promoted podocyte apoptosis, while TAC protected podocyte by inhibiting the nuclear translocation of NFAT.Citation29,Citation30 As Cabin1 undergone nuclear translocation during podocyte injury and positively regulated p53, TAC might protect podocyte via blocking the nuclear translocation of Cabin1.
In this study, Cabin1 protein expression significantly increased and undergone nuclear translocation in the remnant kidney of 5/6 nephrectomized rats, while TAC stabilized the expression and distribution of Cabin1. Cabin1 may become a new target to demonstrate the mechanism of TAC in podocyte injury.
Disclosure statement
The authors declare no competing interests.
Funding information
This study was supported by Science and Technology Planning Project of Guangdong Province, China (grant No. 2011B0, 3180 0307) and Doctoral Program Foundation of Guangzhou Medical University, China (grant no. 2014C33).
References
- Kitamura N, Matsukawa Y, Takei M, Sawada S. Antiproteinuric effect of angiotensin-converting enzyme inhibitors and an angiotensin II receptor blocker in patients with lupus nephritis. J Int Med Res. 2009;37:892–898.
- Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;38:1202–1211.
- Lv J, Zhang H, Chen Y, et al. Combination therapy of prednisone and ACE inhibitor versus ACE-Inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis. 2009;53:26–32.
- Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24:3694–3701.
- Tang S, Leung JC, Chan LY, et al. Mycophe-nolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–812.
- Duncan N, Dhaygude A, Owen J, et al. Treatment of focal and segmental glomerulosclerosis in adults with tacrolimus monotherapy. Nephrol Dial Transplant. 2004;19:3062–3067.
- Loeffler K, Gowrishankar M, Yiu V. Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr Nephrol. 2004;19:281–287.
- Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938.
- Li JS, Chen X, Peng L, et al. Angiopoietin-like-4, a potential target of Tacrolimus, predicts earlier podocyte injury in minimal change disease. PLoS One. 2015;10:e0137049.
- Hoorn EJ, Walsh SB, McCormick JA, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309.
- Ozbay LA, Moller N, Juhl C, et al. Calcineurin inhibitors acutely improve insulin sensitivity without affecting insulin secretion in healthy human volunteers. Br J Clin Pharmacol. 2012;73:536–545.
- Hammond DR, Udvadia AJ. Cabin1 expression suggests roles in neuronal development. Dev Dyn. 2010;239:2443–2451.
- Wen Y, Wang Z, Liu L, Zhang Y, Zhou P, Liang J. Cabin1 localizes in glomerular podocyte and undergoes nuclear translocation during podocyte injury. Ren Fail. 2015;37:1344–1348.
- Wen Y, Zhou P, Liu L, Wang Z, Zhang Y, Liang J. Effect of the knockdown of Cabin1 on p53 in glomerular podocyte. J Recept Signal Transduct Res. 2015;23:1–8.
- Skupien J, Warram JH, Smiles A, Galecki A, Stanton RC, Krolewski AS. Improved glycemic control and risk of ESRD in patients with type 1 diabetes and proteinuria. J Am Soc Nephrol. 2014;25:2916–2925.
- Zhang Q, Shi SF, Zhu L, et al. Tacrolimus improves the proteinuria remission in patients with refractory IgA nephropathy. Am J Nephrol. 2012;35:312–320.
- Li X, Li H, Ye H, et al. Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR. Am J Kidney Dis. 2009;54:51–58.
- Roberti I, Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol. 2010;25:1117–1124.
- Lim SW, Jin L, Piao SG, Chung BH, Yang CW. Inhibition of dipeptidyl peptidase IV protects tacrolimus-induced kidney injury. Lab Invest. 2015;95:1174–1185.
- Liao R, Liu Q, Zheng Z, et al. Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS One. 2015;10:e0132724.
- Shengyou Y, Li Y, Zhihong H, Yuanyuan M. Influence of tacrolimus on podocyte injury inducted by angiotensin II. J Renin Angiotensin Aldosterone Syst. 2015;16:260–266.
- Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2000;97:1196–1201.
- Lai MM, Luo HR, Burnett PE, Hong JJ, Snyder SH. The calcineurin-binding protein cain is a negative regulator of synaptic vesicle endocytosis. J Biol Chem. 2000;275:34017–34020.
- Yi JK, Kim HJ, Yu DH, et al. Regulation of inflammatory responses and fibroblast-like synoviocyte apoptosis by calcineurin-binding protein 1 in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64:2191–2200.
- Wang L, Chang JH, Paik SY, Tang Y, Eisner W, Spurney RF. Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol. 2011;25:1376–1386.
- Fan P, Anthony R, Means J, Liu O. Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation. Embo J. 2005;24:2104–2113.
- Jang H, Choi SY, Cho EJ, Youn HD. Cabin1 restrains p53 activity on chromatin. Nat Struct Mol Biol. 2009;16:910–915.
- Ying CY, Dominguez-Sola D, Fabi M, et al. MEF2B mutations lead to deregulated expression of the oncogene BCL6 in diffuse large B cell lymphoma. Nat Immunol. 2013;14:1084–1092.
- Nijenhuis T, Sloan AJ, Hoenderop JG, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179:1719–1732.
- Zhang B, Shi W, Ma J, et al. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J Mol Med (Berl). 2012;90:1407–1420.
