Abstract
Introduction Cardiovascular disease is an important factor in the mortality and morbidity of patients with end-stage renal disease receiving hemodialysis. Although mineralocorticoid receptor antagonists may have potential benefits on the cardiovascular system, their safety for patients on hemodialysis remains unclear, considering the differences between the results of already performed clinical trials. Methods MEDLINE, EMBASE, Cochrane, ClinicalTrials.gov and PubMed databases were searched for relevant clinical trials. The Cochrane Collaboration assessment tool was employed to evaluate the quality of the randomized controlled trials. Revman 5.3 was used to perform the meta-analysis. Results Eleven studies (n=379) were included in the systematic review and five randomized controlled trials were included in the meta-analysis (n=248). Mineralocorticoid antagonists (MRAs) did not increase predialysis potassium levels significantly (0.11, 95% confidence interval −0.03 to 0.25, p = 0.11). However, the studies included in this review reported inconsistently with respect to effects of mineralocorticoid receptor antagonists on blood pressure, left ventricular ejection fraction and left ventricular hypertrophy, and quantitative analysis was not performed due to insufficient data. One trial showed that the mineralocorticoid receptor antagonists were associated with decreased carotid intima-media thickness and other articles concluded that mineralocorticoid receptor antagonists had no effect on aortic stiffness. Conclusion It is safe to use low dose mineralocorticoid receptor antagonists on patients receiving hemodialysis, at the end of each session of hemodialysis, and close monitoring of serum potassium levels and possible side effects is necessary. The cardiovascular actions still need to be explored and large scale RCTs are in progress.
Introduction
Cardiovascular (CV) disease is an important factor of the mortality and morbidity of patients with end-stage renal disease receiving hemodialysis and accounts for about half of all fatalities.Citation1–3 Hypertension is a common yet frequently uncontrolled complication in hemodialysis,Citation4 which is an important risk factor for left ventricular hypertrophy (LVH), cardiac failure, coronary artery disease (CAD), arrhythmia, and heart failure.Citation5 LVH is highly prevalent in individuals with ESRD and a risk factor for death in the ESRD population, and presence of LVH over time is strongly associated with the risk of death during follow-up.6 Heart failure, defined as a reduction in the left ventricular ejection fraction (LVEF), occurs in a considerable proportion of patients on hemodialysis and impacts morbidity and mortality.Citation7
Aldosterone and mineralocorticoid receptors (MRs) have been recognized for their significant role in potassium excretion and sodium and water retention.Citation8,Citation9 Recent researches have revealed that they are also responsible for hypertension, congestive heart failure and ventricular hypertrophy in patients receiving hemodialysis.Citation6,Citation10–12 To prevent these negative impacts, the inhibition of renin-angiotensin-aldosterone system (RAAS) should be considered in the treatment of patients undergoing hemodialysis. Nevertheless, it has been reported that angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) alone may not effectively inhibit aldosterone production in all patients,Citation7,Citation10 and consequently, the mineralocorticoid antagonists (MRAs) should be considered for complete blockade of RAAS.
MRAs, including spironolactone and eplerenone, have been proven effective in the treatment of patients with heart failure and hypertension.Citation13–15 However, the use of MRAs on patients receiving hemodialysis is still controversial, considering the possibility of an elevated serum potassium level or even fatal hyperkalemia due to a patient’s compromised ability to maintain electrolyte balance.Citation16 Therefore, we performed this systematic review and meta-analysis to evaluate the effect of MRAs on the serum potassium level and CV system for patients undergoing hemodialysis.
Methods
Search strategy
We searched MEDLINE, EMBASE, Cochrane, ClinicalTrials.gov and PubMed databases for relevant literature until August 2015. The following terms were used for searching: MR antagonist, MR blocker, aldosterone receptor blocker, aldosterone receptor blocker, aldosterone blocker, spironolactone, eplerenone, canrenoate, hemodialysis, extracorporeal dialysis, renal replacement therapy, renal dialysis, ESRD, renal failure, kidney failure and end-stage kidney disease. Additionally, we searched articles in magazines by hand as a supplement and contacted the authors for unpublished data if necessary. All of the searches were limited to human studies. There was no restriction regarding language.
Inclusion and exclusion criteria
Type of studies. Randomized controlled trials (RCTs) or clinical trials in which (a) an intervention arm and a control arm were designed or (b) participants served as their own control. Crossover studies were also accepted if there was evidence of a sufficient washout period without carry-over effect. Only RCTs were included for the meta-analysis.
Subjects. Participants were on hemodialysis for at least 1 month with predialysis serum potassium <6.5 mmol/L. Patients with a history of kidney transplantation were excluded.
Interventions. Spironolactone or eplerenone administered for at least 2 weeks.
Outcome. The primary outcome was the serum potassium level and the secondary outcome was CV effects.
Quality assessment
The assessment tool developed by the Cochrane CollaborationCitation17 was applied to evaluate all potentially relevant sources of bias, including the selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases of RCTs. The assessment was performed by two independent authors and disagreements were resolved by discussion with a third author.
Funding source of included RCTs
As recommended by Roseman et al.,Citation18 funding sources of included RCTs were reported.
Data extraction
The data were extracted independently by two different investigators using a collection form that we designed (Supplementary data 1). Data presented only in graphs and figures were extracted whenever possible, but they were included only if the two reviewers had the same results. Data not published were acquired by contact with the original investigators and, if that failed, calculated with available data.
Statistical analysis
Considering the high potential risk of bias in non-RCTs, we only included RCTs in the meta-analysis. We employed Revman 5.3 software (the Cochrane Collaboration, UK)Citation19 to perform the meta-analysis to estimate the serum potassium level after intervention. The results were presented as the mean difference with 95% confidence intervals. Additionally, we calculated the Chi square and I2 values to evaluate the heterogeneity among the studies according to the Cochrane Handbook.Citation20
Results
Search results
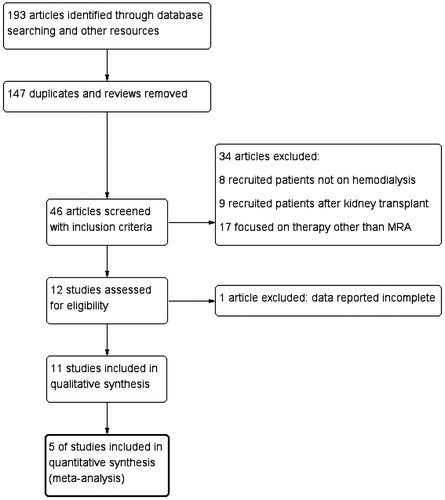
A total of 193 articles were retrieved, among which 147 were duplicates or reviews. Forty six articles were screened based on the titles and abstracts, and 12 studies met the criteria for inclusion. After full-text screening, one article was excluded as no data of serum potassium for the control group was provided.Citation21 Eleven studies were reported in this systematic review, among which five studies were RCTsCitation22–26 and six were non-randomized studiesCitation27–32 ().
Characteristics of the included trials
The characteristics of the included trials are summarized in . Five studies were RCTs, one of which was a crossover study. Six were prospective non-randomized studies, three of which assigned a placebo group as the control, and the other studies considered the participants at baseline as their own control. All of the trials reported the serum potassium level before dialysis. Blood pressure, LVEF, left ventricular mass (LVM) and other parameters used for assessing the CV effects were reported in seven articles.
Table 1. Summarization of included studies.
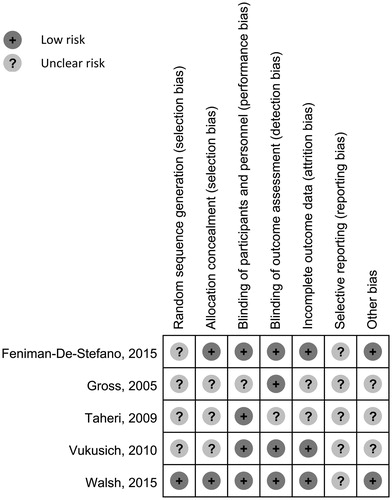
All potentially relevant sources of bias of included RCTs were evaluated and are demonstrated in . It should be noted that, due to the lack of the original study protocol and insufficient information provided in the literatures, most sources of bias were assessed as ‘Unclear”. No particular source of bias was present in the studies reviewed.
Figure 2. Risk of bias assessment. Potential risks of bias are assessed using the assessment tool developed by the Cochrane CollaborationCitation17 and are presented.
Funding sources of included RCTs
The RCT conducted by Feniman-De-Stefano et al.Citation25 was funded by FUNDUNESP (Foundation for the Development of UNESP, Process 0090910) and FAPESP (Foundation for Research Support of São Paulo, Process 2010/10439–1). The authors declared no conflicts of interests.
The RCT conducted by Walsh et al.Citation26 was funded by Canadian Institutes of Health Research, the Canadian Network and Center for Trials Internationally, the Canadian Kidney Knowledge Translation and Generation Network, and the Pfizer Investigator Initiated Research program. No funder had any role in the design, conduct, or reporting of the trial. One of the authors was supported by a New Investigator Award from the Kidney Research Scientist Core Education and National Training (KRESCENT) Program, and received research funding for this study from Pfizer through the Investigator Initiated Research program at Pfizer.
The RCT conducted by Vukusich et al.Citation24 was supported by grants FONDECYT 1040338 and Fondo Ayuda Investigación Universidad Los Andes MED 002/06.
The funding sources of the RCT conducted by GrossCitation22 and the RCT by Taheri et al.Citation23 were not disclosed.
Effect of MRA on the predialysis potassium level
Reported serum potassium data are summarized in . The dose of the MRAs administered varied dramatically and the serum potassium level changed accordingly.
Table 2. Effect of mineralocorticoid receptor antagonists on serum potassium level.
Among the studies included in our review, eight studies reported no significant change in the serum potassium level. Saudan et al.Citation27 enrolled 35 patients who were treated with either placebo or sequentially 12.5 mg of spironolactone thrice weekly for 2 weeks and 25 mg of spironolactone thrice weekly for 2 weeks. The serum potassium level at study completion was 4.9 ± 0.3 in the spironolactone group and 4.9 ± 0.7 in the control group, with no significant difference. Hussain et al.,Citation31 Shavit et al.,Citation29 Michea et al.,Citation28 Gross et al.,Citation22 Vukusich et al.,Citation24 Feniman-De-Stefano et al.Citation25 and Walsh et al.Citation26 also found no significant change in the serum potassium level. These trials used relatively small doses of MRAs (no more spironolactone or eplerenone than 25 mg daily).
Other investigators reported different results. Matsumoto et al.Citation32 reported that after administration of spironolactone at a dose of 25 mg daily for 6 months, there was a significant rise in serum potassium after spironolactone treatment (5.18 ± 0.72 vs. 4.96 ± 0.72 mmol/L, p < 0.05). Taheri et al.,Citation23 Flevari et al.Citation30 and Gross et al.Citation22 reported a significant rise in the serum potassium level after administration of MRAs. These investigators administered MRAs at larger doses (no less than 50 mg thrice a week).
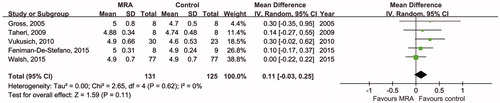
To evaluate the influence of MRA comprehensively, data from five RCTs were pooled to provide an overall estimate of the predialysis potassium level after intervention (). There was no significant difference between the MRA group and the placebo group (mean difference 0.11, 95% CI −0.03, 0.25 mmol/L, p = 0.11), and there was low heterogeneity (Chi2=2.65, p = 0.62, I2 = 0%).
Other side effects of MRA
Several side effects of MRAs were reported, including gastrointestinal side effects, dizziness, hyperemia, gynecomastia, dry mouth, nose bleed, mild pruritus, gynecomastia, leg numbness, sleepiness and unpleasant feelings. Only eleven patients withdrew from the study because of these side effects.Citation31,Citation32
CV effects of MRA
The outcome of the CV effects explored in the trials was blood pressure, parameters of ventricular hypertrophy, carotid intima-media thickness (CIMT) and pulse wave velocity (PWV). A meta-analysis of these outcomes was impossible due to insufficient subjects from the RCTs.
Blood pressure (BP)
BP was measured and reported in six trials, fiveCitation22,Citation24,Citation25,Citation29,Citation30 of which provided systolic blood pressure (SBP) and diastolic pressure (DBP), and the other trial reported the change of BP after interventionCitation26 (). Patients with hypertension and receiving antihypertensive treatment continued their therapy during the trial. Gross and colleaguesCitation22 reported a significant decrease of SBP after spironolactone administration (131.4 ± 18.2 vs. 142.0 ± 19.6, p < 0.05), while there was no significant difference in DBP. Shavit et al.Citation29 also noticed a significant drop in the SBP after eplerenone administration (from 166 ± 14 to 153 ± 10, p<0.021), but no significance in the DBP was observed. In the sequential placebo-controlled study conducted by Flevari et al.,Citation30 there were significant differences in both the SBP and DBP in the spironolactone group and placebo group (121 ± 8.6/66 ± 2.5 vs. 147 ± 13.8/76 ± 2.6, p < 0.05). Other investigators reported no significant effect of MRA on BP.
Table 3. Effect of mineralocorticoid receptor antagonists on blood pressure.
Left ventricular ejection fraction
Taheri et al.Citation23 evaluated the cardiac function of hemodialysis patients treated with spironolactone. Compared with the placebo group, LVEF improves significantly after spironolactone administration (6.2%±1.6% vs. 0.8%±4.9%, p = 0.046). Feniman-De-StefanoCitation25 and colleagues observed different results. No significant difference in the LVEF between the spironolactone group and placebo group was observed (70.9%±4.2% vs. 69.7 ± 5.2, p = 0.89).
Left ventricular hypertrophy
LVH can be evaluated by LVM, left ventricular mass index (LVMI), and thickness of the left ventricular walls. In the study conducted by Taheri and his team,Citation23 the change in the LVM of the spironolactone group was significantly different from the placebo group (−8.4 g/m2±4.72 vs. 3 g/m2±7.97 g/m2, p = 0.021). Feniman-De-StefanoCitation25 reported similar results (LVM at the end of study, 236 g ± 36.1 g vs. 273 g ± 65.5 g, p = 0.046). Their study also showed a significant decrease in posterior wall thickness in the spironolactone group compared with the placebo group (11.9 mm ± 0.8 mm vs. 12.7 mm ± 1.0 mm, p = 0.043). Flevari et al.Citation30 found different results in their study, which showed no difference in the LVM and left ventricular wall thickness after treatment with a placebo and spironolactone (placebo: 251 g ± 15 g, spironolactone: 250 g ± 18 g, no significant difference).
Carotid intima-media thickness
CIMT was measured in the trial performed by Vukusich et al.,Citation24 which showed a significant decrease in intima-media thickness in the common carotid artery, carotid bifurcation and internal carotid artery.
Aortic stiffness
Feniman-De-StefanoCitation25 examined the PWV of participants and reported no significant difference (spironolactone group: 10.1 m/s ± 4.5 m/s, placebo group: 11.7 m/s ± 4.6 m/s, p < 0.560).
Discussion
The safety of MRAs for patients receiving hemodialysis has been a controversial topic with two systematic reviews already published.Citation33,Citation34 A clear conclusion regarding safety and its positive effect, however, remains unclear.
Although the overall conclusion of the quantitative analysis suggests that the MRA exerts no significant effect on the predialysis serum potassium level compared with the placebo, the differences between the results should be explored. The studies that reported no significant difference between the MRA and placebo group used a rather low-dose of the MRA (no more than 25 mg daily of spironolactone or eplerenone).Citation25–29,Citation31 In the trials that found a significant increase in the serum potassium level after administration of MRAs, large doses (50 mg thrice a week) of MRAs were used.Citation23,Citation24,Citation30,Citation32 There was an exception, the trial conducted by Gross et al.,Citation22 in which patients took spironolactone 50 mg twice daily for 2 weeks, and no significant increase in the serum potassium level was observed. The underlying explanation for this may lie in the exclusion criteria – patients using ACEI or ARB were excluded, therefore lowering the potential effect on the serum potassium level. Interestingly, Ng et al. found in their meta-analysis of 28 studies that the use of MR antagonists was associated with increased serum potassium and a higher risk ratio of hyperkalemia.Citation35 A possible explanation is that Ng included patients with chronic kidney disease (CKD) of stage I to stage V, while this review only included patients receiving hemodialysis. For patients with CKD of stage I–IV, residual renal function is the main approach for potassium excretion. In patients with ESRD and undergoing hemodialysis, however, regular hemodialysis plays the most important role in potassium balance, and by adjusting the potassium concentration in the dialysate, the serum potassium level can be controlled more easily.Citation36
The incidence of hyperkalemia was reported in a few studies with little consistency. Only Walsh et al.Citation26 reported a significant increase in the incidence rate of hyperkalemia in the MRA group, and that might have been the result of increasing the dose of eplerenone during the trial. Nevertheless, it must be noted that the subjects included in all of the trials had no previous history of hyperkalemia and remained compliant to therapy. Meanwhile, we need to learn a lesson from the RALES studies. After the results were published, the prescription rate rose significantly and the incidence of hyperkalemia was much higher than that reported in the studies,Citation37,Citation38 indicating the necessity of surveillance of the serum potassium level. Additionally, an appropriate timetable for administration of MRAs is crucial to avoid hyperkalemia, and particularly, the relationship between MRAs and dialysis and meal must be considered. In three RCTs,Citation23–25 MRAs were administered after each dialysis, when the serum potassium level was relatively low. Noori et al. found that the more potassium intake was associated with higher serum potassium level.Citation39 Consequently, the ideal window for MRAs administration may be at the end of the dialysis and far away from meals to avoid hyperkalemic episodes. Conclusively, we may safely consider using MRAs on patients receiving hemodialysis at a low dose at the end of the dialysis and far away from meal, but serum potassium levels need to be monitored closely, and the patients should be cautiously observed for potential side effects, which can be easily accomplished due to the regular visits to the hospital or dialysis center.
Hypertension is the initial factor of other severe CV conditions, including LVH, arterial damage, cardiac ischemia, arrhythmia and, ultimately, heart failure,Citation5 and therefore controlling blood pressure is critical in the management of patients on hemodialysis. It is suggested by three studiesCitation22,Citation29,Citation30 that there was a significant drop in blood pressure after the administration of MRAs. In these studies, relatively large doses of spironolactone were used (from 25 mg twice daily to 50 mg twice daily). Those studies that found no significant drop in BP used rather low dose of MRAs (no more than 50 mg thrice weekly).Citation24–26 It is possible that MRAs may serve an important part in the future treatment paradigm, provided that the dose is accurately titrated.
LVH, as the consequence of increased afterload, humoral and cellular factors, altered myocardial metabolism and rheostatic factors, progresses rapidly without intervention and is an important risk factor in end-stage renal disease. Taheri and Feniman-De-StefanoCitation23,Citation25 observed a decrease in the LVM after low doses of spironolactone. Flevari et al.Citation30 found that spironolactone had no effect on the LVM, which may be explained by the fact that only patients without heart failure or hypertension and, therefore, a lower possibility of LVH, were included. If future studies confirm the necessity of intervention, low-dose MRA might be considered as a potential choice. However, whether targeting LVH is appropriate is still uncertain. Despite the promising results of several trials, whether therapeutic improvement in LVMI reduces mortality remains unclear.Citation6
Heart failure, the eventual outcome of all CV events, volume overload between dialyses and anemia,Citation40 presents primarily as a decrease in the LVEF. Despite the fact that MRAs have already been proven effective in the treatment of heart failure in patients without kidney disease,Citation13,Citation41 whether they are beneficial for patients on hemodialysis and the ‘safe dose’ remains to be determined. We suppose the difference between the results from Taheri and Feniman-De-Stefano may derive from the difference between the inclusion criteria. Taheri only enrolled patients with a LVEF no more than 45%,23 but Feniman-De-Stefano put no restriction on LVEF.25 These trials were not quite convincing, considering the small sample size. Large-scale trials are needed.
Elevated CIMT is considered as an indicator of atherosclerosis.Citation42,Citation43 The trial conducted by Vukusich et al. showed that MRAs had the potential to reduce CIMT and decrease the incidence of atherosclerosis and further CV thromboembolic events, including myocardial infarction and stroke.Citation24
Most importantly, the effect of MRAs on the mortality of hemodialysis patients remains to be explored. We are pleased that such studies are beginning. Matsumoto et al.Citation21 conducted a trial focusing on the mortality and morbidity rate of CV and cerebrovascular (CCV) events of patients on hemodialysis treated with spironolactone. Three hundred and nine patients were recruited, 157 for the spironolactone group and 152 for the placebo group. The death from CCV causes, hospitalization for CCV causes and the death from all causes decreased significantly. However, the article failed to provide the serum potassium level and was therefore excluded from the review. Indeed, this was one trial with a relatively small sample size, yet it marked the first step on the way to full comprehension of the effects of MRAs on hemodialysis patients.
The MRAs administered in the included trials were spironolactone or eplerenone, both of which have been proved efficacious in the treatment of heart failure and hypertension.Citation44 Compared to spironolactone, eplerenone is a selective aldosterone antagonist with lower incidence of sexual side effects due to lower affinity for progesterone and androgen receptors.Citation45 However, highly polymorphic cytochrome dependent metabolism makes eplerenone susceptible to drug interactions.Citation46 Besides, the cost of eplerenone is significantly higher than that of spironolactone (approximately $113 vs. $24 per months) since spironolactone has been off-patent.Citation45 Regretfully, there have been no trials comparing spironolactone and eplerenone in patients on hemodialysis. In clinical practice, the comorbidities of patients and economic conditions should be considered during the decision making of treatment plans.
Meanwhile, the non-steroidal MR antagonists are being evaluated, finerenone being one of them, which has been tested through phase I to phase IIb. The Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS)Citation47–49 included patients with heart failure and CKD. It was reported that finerenone decreased the concentration of NT-proBNP and BNP. Additionally, although not statistically significant, there was a trend that finerenone was associated with lower urinary albumin to creatinine ratio. Compared to spironolactone, finerenone did not decrease eGFR and increase serum potassium level. Considering the above advantages, finerenone may be a promising candidate MRA in the treatment of patients undergoing dialysis, especially those with heart failure.
It must be noted that this review is a preliminary summarization of clinical trials. The methodology of some trials may increase the risk of bias, especially considering that only five studies were RCTs and the others were not designed to be double-blinded and did not have a parallel control arm as a comparison. Additionally, each study recruited only a limited number of patients and, therefore, the number of patients included in this review is relatively small (379 for systematic review and 248 for quantitative analysis). Meanwhile, the inclusion criteria, study design and intervention method of different studies were not identical, which is a possible source of heterogeneity in the meta-analysis. Large-scale multi-center RCTs are necessary to provide further evidence for the safety and CV benefits of MRAs on patients receiving hemodialysis.
Notably, at least three RCTs investigating the effect of MRAs on patients undergoing hemodialysis are already in process. Hammer et al.Citation50 conducted a prospective, randomized, placebo-controlled, double-blind, parallel group, multi-center intervention study (NCT01691053) to investigate the effects of spironolactone (50 mg daily) compared with placebo in maintenance hemodialysis patients. The outcomes include pre-dialysis potassium levels, incidence of life-threatening hyperkalemia, LVMI, changes in left ventricular geometry and function, 24-h ambulatory blood pressure, cardiac arrhythmias, vascular function parameters, measures of heart failure and quality of life. We are optimistic that with the accumulation of high-quality large-scale RCTs, there will be a sophisticated guide for the use of MRAs in patients receiving hemodialysis. Currently, it is reasonable to use low-dose MRAs safely with cautious monitoring of the serum potassium level, and its potential effect on blood pressure, LVEF and LVH. A second ongoing RCT by Central Hospital and Institut National de la Santé Et de la Recherche Médicale of FranceCitation51 (NCT01848639) is recruiting patients undergoing chronic hemodialysis to investigate the effect of spironolactone (25 mg per 2 days, after dialysis). The primary outcome is the time to onset of the first non-fatal MI or acute coronary syndrome or hospitalization for heart failure or nonfatal stroke or CV death, and other outcomes include incidence of hyperkalemia, the cumulative rate of non-fatal MI or acute coronary syndrome, hospitalization for heart failure, nonfatal stroke or CV death, etc. Another similar RCT by Brigham and Women’s Hospital is also recruiting participants (NCT02285920).Citation52
Disclosure statement
The authors report no conflict of interest.
References
- Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Renal Care. 2010;36:4–8.
- Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl. 2015;5:2–7.
- Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;65:A7.
- Sinha AD, Agarwal R. Peridialytic, intradialytic, and interdialytic blood pressure measurement in hemodialysis patients. Am J Kidney Dis. 2009;54:788–791.
- Zoccali C, Benedetto FA, Tripepi G, Mallamaci F. Cardiac consequences of hypertension in hemodialysis patients. Semin Dial. 2004;17:299–303.
- Charytan D. Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23:578–585.
- K/DOQI Workgroup: K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–153.
- Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: Past, present, and future. Endocrinology. 2010;151:5098–5102.
- Funder JW. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog Cardiovasc Dis. 2010;52:393–400.
- Shavit L, Lifschitz MD, Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: Current concepts and emerging treatment paradigms. Kidney Int. 2012;81:955–968.
- McLaughlin N, Gehr TW, Sica DA. Aldosterone-receptor antagonism and end-stage renal disease. Curr Hypertens Rep. 2004;6:327–330.
- Agrawal S, Agrawal N, Garg J, Mohandas R, Gupta T, Segal M. Heart failure and chronic kidney disease: Should we use spironolactone? Am J Med Sci. 2015;350:147–151.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717.
- Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
- Stewart Coats AJ, Shewan L. Eplerenone's role in the management of complex cardiovascular disorders. Int J Cardiol. 2015;200:1–2.
- Pitt B, Rossignol P. Mineralocorticoid receptor antagonists in patients with end-stage renal disease on chronic hemodialysis. J Am Coll Cardiol. 2014;63:537–538.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed). 2011;343:d5928.
- Roseman M, Milette K, Bero LA, et al. Reporting of conflicts of interest in meta-analyses of trials of pharmacological treatments. JAMA. 2011;305:1008–1017.
- Buljan M, Nemet D, Golubic-Cepulic B, Bicanic G, Tripkovic B, Delimar D. Two different dosing regimens of human recombinant erythropoietin beta during preoperative autologous blood donation in patients having hip arthroplasty. Int Orthopaed (SICOT). 2012;36:703–709.
- Kikuchi H, Tan A, Nonaka T, Shimada W, Tanaka S. Comparison of intravenous and subcutaneous erythropoietin therapy for preoperative acquisition of blood for autologous transfusion in patients undergoing total arthroplasty. J Orthop Sci. 1997;2:84–87.
- Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–536.
- Gross E, Rothstein M, Dombek S, Juknis HI. Effect of spironolactone on blood pressure and the renin–angiotensin–aldosterone system in oligo-anuric hemodialysis patients. Am J Kidney Dis. 2005;46:94–101.
- Taheri S, Mortazavi M, Shahidi S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transplant. 2009;20:392–397.
- Vukusich A, Kunstmann S, Varela C, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1380–1387.
- Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Therapeut Adv Cardiovasc Dis. 2015;9:158–167.
- Walsh M, Manns B, Garg AX, et al. The safety of eplerenone in hemodialysis patients: A noninferiority randomized controlled trial. Clin J Am Soc Nephrol. 2015;10:1602–1608.
- Saudan P, Mach F, Perneger T, et al. Safety of low-dose spironolactone administration in chronic hemodialysis patients. Nephrol Dial Transplant. 2003;18:2359–2363.
- Michea L, Vukusich A, Gonzalez M, Zehnder C, Marusic ET. Effect of spironolactone on K(+) homeostasis and ENaC expression in lymphocytes from chronic hemodialysis patients. Kidney Int. 2004;66:1647–1653.
- Shavit L, Neykin D, Lifschitz M, Slotki I. Effect of eplerenone on blood pressure and the renin–angiotensin–aldosterone system in oligo-anuric chronic hemodialysis patients—A pilot study. Clin Nephrol. 2011;76:388–395.
- Flevari P, Kalogeropoulou S, Drakou A, et al. Spironolactone improves endothelial and cardiac autonomic function in non heart failure hemodialysis patients. J Hypertens. 2013;31:1239–1244.
- Hussain S, Dreyfus DE, Marcus RJ, Biederman RW, McGill RL. Is spironolactone safe for dialysis patients? Nephrol Dial Transplant. 2003;18:2364–2368.
- Matsumoto Y, Kageyama S, Yakushigawa T, et al. Long-term low-dose spironolactone therapy is safe in oligoanuric hemodialysis patients. Cardiology. 2009;114:32–38.
- Baker WL, White WB. Safety of mineralocorticoid receptor antagonists in patients receiving hemodialysis. Ann Pharmacother. 2012;46:889–894.
- Chua D, Lo A, Lo C. Spironolactone use in heart failure patients with end-stage renal disease on hemodialysis: Is it safe? Clin Cardiol. 2010;33:604–608.
- Ng KP, Arnold J, Sharif A, Gill P, Townend JN, Ferro CJ. Cardiovascular actions of mineralocorticoid receptor antagonists in patients with chronic kidney disease: A systematic review and meta-analysis of randomized trials. J Renin–Angiotensin–Aldosterone Syst. 2015;16:599–613.
- Choi HY, Ha SK. Potassium balances in maintenance hemodialysis. Electrolyte Blood Press Blood. 2013;11:9–16.
- Hauben M, Reich L, Gerrits CM, Madigan D. Detection of spironolactone-associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES). Drug Saf. 2007;30:1143–1149.
- Beizer JL. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. Consult Pharmacist. 2005;20:148–149.
- Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56:338–347.
- Malik J, Tuka V, Mokrejsova M, Holaj R, Tesar V. Mechanisms of chronic heart failure development in end-stage renal disease patients on chronic hemodialysis. Physiol Res/Acad Sci Bohemoslov. 2009;58:613–621.
- Eschalier R, McMurray JJ, Swedberg K, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–1593.
- Tsivgoulis G, Vemmos K, Papamichael C, et al. Common carotid artery intima-media thickness and the risk of stroke recurrence. Stroke. 2006;37:1913–1916.
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437.
- Lainscak M, Pelliccia F, Rosano G, et al. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol. 2015;200:25–29.
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–158.
- Seferovic PM, Pelliccia F, Zivkovic I, et al. Mineralocorticoid receptor antagonists, a class beyond spironolactone—Focus on the special pharmacologic properties of eplerenone. Int J Cardiol. 2015;200:3–7.
- Pitt B, Filippatos G, Gheorghiade M, et al. Rationale and design of ARTS: A randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail. 2012;14:668–675.
- Pitt B, Anker SD, Bohm M, et al. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): A randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. none. in who worsening heart with andor chronic disease. Eur J Heart Fail. 2015;17:224–232.
- Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463.
- Hammer F, Krane V, Stork S, et al. Rationale and design of the Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease Study (MiREnDa). Nephrol Dial Transplant. 2014;29:400–405.
- University Hospital, Brest. ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST). 2013. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01848639. Accessed January 13, 2016.
- University of Pennsylvania. Safety and Cardiovascular Efficacy of Spironolactone in Dialysis-Dependent ESRD Trial (SPin-D). 2014. Available at: https://www.clinicaltrials.gov/ct2/show/NCT02285920.