Abstract
Objectives Diabetic nephropathy (DN) is the leading cause of end-stage renal disease worldwide. The NO system has been implicated in the pathogenesis of DN. In this study, we aimed to evaluate the healing effect of pentoxifylline on NOS in STZ-induced diabetic rat’s kidney. Material and methods In this study, 50 Wistar albino male rats were used. The rats were divided into five groups; Group C control; Group D only diabetes; Group D + PI and D + PII diabetes + pentoxifylline; Group P only pentoxifylline. Group DPI rats received just pentoxifylline from the beginning of the experiments. However, Group DPII rats received saline in the first month and 50 mg/kg/day of pentoxifylline for the following month. At the end of two months, NOS expressions in kidney tissue were assessed using qRT-PCR and immunohistochemistry analysis. Results At the end of the experiments, desquamation of the epithelial cells of the tubules, clear glycogen-filled distal tubules and increased number of apoptotic cells were seen in Group D. Diabetic rats’ nNOS immunoreactivity had increased and eNOS and iNOS immunoreactivity had decreased; nNOS, iNOS and eNOS mRNA levels tended to decrease compared to the control group. PTX ameliorated eNOS, iNOS and nNOS protein levels and apoptotic cells, but did not affect mRNA levels. Conclusion In conclusion, PTX has a healing effect on this damage by affecting NOS expression.
Introduction
Diabetes Mellitus (DM) is known to be one of the most serious diseases threatening the human health in the modern world. Unfortunately, according to the World Health Organization forecasts, the number of diabetes patients will reach up to 366 million in the world in 2030.Citation1 Up to 40% of patients with diabetes will develop some form of kidney damage. Diabetes has become the most common single cause of end-stage renal disease (ESRD) worldwide. Diabetic nephropathy (DN) is the leading cause of ESRD.Citation2
A high glucose level is responsible for the diabetes induced damages in the body. As a result, long-lasting hyperglycemia causes cell damage via increased glucose oxidation, glycolysis, polyol pathway formation, glycation end product activation and nitric oxide (NO) production.Citation3 The NO system has been implicated in the pathogenesis of DN. NO is a free radical gaseous molecule with a biological half-life of a few seconds. NO is generated from the guanidine nitrogen of l-arginine by three isoforms of nitric oxide synthase (NOS), that is, neuronal NOS (nNOS, NOS-1), endothelial NOS (eNOS, NOS-3), and inducible NOS (iNOS, NOS-2). NO acts as an intracellular and intercellular messenger in different tissues.Citation4 It has numerous functions in the kidney and all three NOS isoforms are expressed in the kidney under physiological and diabetic conditions.Citation5 Hyperglycemia can lead to endothelial dysfunction. This may result from decreased production of NO.Citation6 Previous studies reported that the development and progression of chronic renal failure in diabetes play a role in the chronic inhibition of NO.Citation7 However, the underlying mechanisms by which changes in the NO level are related to the renal hemodynamics and morphology in DM have not been fully elucidated.
Pentoxifylline (PTX), a nonspecific phosphodiesterase inhibitor, was first considered for use in the treatment of peripheral vascular diseases.Citation8 PTX exerts several pharmacologic effects, including improvement in microcirculation, increase in erythrocyte deformability, reduction in blood viscosity, inhibition of platelet aggregation, endothelium-dependent vascular relaxation, immunomodulatory, anti-inflammatory, and antiproliferative effects.Citation8 In addition, it has been used as an antioxidant in order to heal damage in several tissues.Citation9 Previous studies reported that PTX ameliorates the clinical markers of glomerular and tubulointerstitial injury in diabetic patients.Citation10 In addition, recently reported studies indicate that PTX may decrease proteinuria. Thus, it has been proposed that PTX could offer some beneficial effects on renal function in patients with DN.
There is a growing body of evidence implicating excessive production of ROS and reactive nitrogen species (RNS) in the metabolic and hemodynamic abnormalities found in DN.Citation5 In vivo studies have demonstrated the beneficial effects of PTX in different models of kidney disease. Therefore, the objective of the present study was to evaluate the healing effect of PTX on NOS in STZ-induced type 1 diabetic rat’s kidney.
Material and methods
Fifty sexually mature male, 8 weeks old, Wistar rats which were obtained from the Hakan Çetinsaya Experimental and Clinical Research Center, Erciyes University, Kayseri, Turkey, were used for this study. The study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Erciyes of approval (Permit Number: 12/32). The rats were randomly assigned to five groups of 10 rats per group. Group C: served as control; Group D: diabetic; Group D + PI: diabetic + PTX; Group D + PII: diabetic + PTX; Group P: PTX administered to rats.
Type 1 diabetes was induced by an intraperitoneal injection of 40 mg/kg body weight of freshly prepared STZ (sc-200719, Santa Cruz Biotechnology, CA). Induction of diabetes was confirmed 72 h later by measuring the tail blood glucose concentration by glucometer (Medisign MM800). Animals with fasting blood glucose levels of >250 mg/dL were included in the study. PTX (Trental 100 mg/5 mL, Sanofi Aventis, Paris, France) 50 mg/kg/day was administered by intraperitoneal injection to the rats. Group D + PI rats were administered PTX from the beginning of the experiment. Group D + PII rats received saline for the first month and underwent a PTX injection during the subsequent month. The control group subjects were administered the same amount of intraperitoneal saline.
At the end of the second month, before the rats were decapitated, their weights and blood glucose levels (from their tails) were measured again. Then the subjects were decapitated under ketamine (75 mg/kg)+xylazine (10 mg/kg) anesthesia; their blood was collected and kidney tissue was removed and weighed. Some of the kidney tissues were used for histological examination, while some were stored in Trizol for RT-PCR. For histological procedure, kidney tissue fixed with 4% (w/v) neutral formaldehyde (cat no: 1039992500, Merck, Darmstadt, Germany) solution for 48 h. Tissues then were washed overnight in flowing tap water. Specimens were dehydrated by immersion in increasing concentrations of alcohol, and then cleared with multiple xylene washes. Tissue blocks then were embedded in paraffin wax. Sections (5 − 6 μm thick) were cut from the paraffin blocks by rotary microtome (Leica RM2155) and were stained with hematoxylin (HX002817, Merck, Darmstadt, Germany) and eosin (cat. no: 1024390500, Merck, Darmstadt, Germany) (H & E) to evaluate general histological structure using an Olympus BX51 light microscope (Center Valley, PA).
Immunohistochemistry
Expressions of eNOS, iNOS and nNOS were detected immunohistochemically in the kidney using a rabbit polyclonal antibody and the streptavidin-biotin peroxidase technique as previously described.Citation11 For this, 5–6 μm sections were taken and kept at 60 °C for one night, then, first all tissues were rehydrated with xylene and afterwards by passing through a graded series of alcohol. Then, 10 mM phosphate buffer saline (PBS) was used to wash the sections, 3× for 5 min. For antigen retrieval, all sections were incubated in 0.01 M sodium citrate buffer, in a microwave oven at 350 W for 3× for 5 min. All sections were then cooled in the same buffer at room temperature for 20 min. Sections were washed again with PBS, and to prevent endogenous peroxidase activity, they were treated with 3% hydrogen peroxide (H2O2) for 5 min. For the next stages, the ABC staining system (Santa Cruz, sc-2023) by means of a coloring kit was used. All cross sections were washed with PBS, and then to make sure to block outside the antigenic fields, block serum was applied for 20 min at room temperature. Then, the sections were immediately incubated with eNOS (Pierce antibody product, PA3-031A, 1/250 dilution), iNOS (Pierce antibody product, PA3-030A, 1/200 dilution) and nNOS (Pierce antibody product, PA3-032A, 1/200 dilution) primary antibodies overnight at +4 °C. As negative control, PBS was used instead of primary antibody. After the washing process, sections were incubated with biotinylated secondary antibody for 30 min and then the washing process was repeated. Then sections were treated with enzyme bracket of Avidin-Biotin (AB) for 30 min. Afterwards, they were washed and to make the immunoreactivities visible a peroxidase substrate, which has diaminobenzidine (DAB) properties, was used for incubation for 5 min and washed with deionized H2O for 5 min. Sections counter-stained with Gill hematoxylin were washed several times with deionized H2O. As the last step, a graded alcohol series was used to remove water from the sections, they were then passed through xylene and sections were finally covered with an entellan. Under the light microscope (Olympus BX51) and using digital camera (DP71), the images were obtained. From each of the subjects, five different areas were evaluated in terms of the expression differences by using the Image J program.
Apoptosis (TUNEL)
The TUNEL method was used to show apoptosis of kidney tissue as previously described.Citation12 An in situ Cell Death Detection Kit, Fluorescein’ Kit (Roche) was used. For the process, first 5–6 μm thick kidney tissues were obtained and after being deparaffinized and rehydrated, they were washed with PBS. After washing for antigen retrieval, the tissues were placed in a 0.01 M sodium citrate buffer in the microwave oven at 350 W for 5 min. Then, they were left to cool for 20 min at room temperature. Having been washed with PBS 3 × for 5 min, the tissues were then incubated with a TUNEL reaction mixture in a damp and dark place at 37 °C for 60 min. After washing with PBS 3× for 5 min, the tissues were then contrast colored with 4,6-diamidine-2′-phenylindole. After covering the tissues with a solution containing glycerol, they were all examined with the Olympus BX – 51 fluorescent microscope at 450–500 nm wavelength. In order to estimate the apoptotic index, TUNEL-positive cells in kidney tissue in 10 randomly chosen fields from each section were counted.
Analysis of gene expression with qRT-PCR
Total RNA was isolated from the 20–30 mg kidney tissues using an MO Bio kit (MO Bio, Solana Beach, CA, cat.:15000–50) following the manufacturer’s protocol. Reverse transcription was performed using hexamer and oligo-dT primers and Transcriptor High Fidelity Reverse Transcriptase (Roche, REF: 05081955001; Version 6.0) following the manufacturer’s protocol (1 μg of isolated RNA per reaction). The acquired cDNA was subjected to quantitative RT-PCR reaction using a LightCycler® 480 Probes Master (Roche, REF: 04707494001; Version 09) and detected with Universal ProbeLibrary Probes (Roche, Applied Sciences, Manheim, Germany) for the iNOS, eNOS and nNOS mRNA and housekeeping genes (). UPL probes are labeled at the 5′ end with fluorescein (FAM) and at the 3′ end with a dark quencher dye. UPL assays are compatible with all real-time PCR instruments capable of detecting fluorescein (FAM) or SYBR Green I. The probes for iNOS, eNOS and nNOS mRNA were labeled with the FAM dye. Amplification of rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and rat beta actin (Actb) were used as housekeeping/reference gene for the quality and endogenous normalization of the RNA samples investigated. The GAPDH and Actb were labeled with FAM dye, so that amplifications were carried out in the same reactions as for iNOS, eNOS and nNOS amplification. Reaction was conducted on a LightCycler® 480 II (Roche) in 45 cycles and the reaction results were analyzed via the efficiency corrected advanced relative quantification algorithm on LightCycler® 480 SW 1.5 software. The conditions of the reaction were as follows: denaturation 95 °C 5 min, annealing of primers 50 °C 15 s, elongation 72 °C 10 s. All samples were tested in duplicate.
Table 1. Sequences of primers and ID numbers of UPL probes used for qRT-PCR.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (SPSS for Windows, SPSS Inc, Chicago, IL, version 15.0). The Kolmogorov–Smirnov test was used to identify normal distribution of the data. In case of normal distribution, quantitative variables were compared using one-way analysis of variance (ANOVA) and post hoc Tukey test. These differences were considered significant when probability was less than 0.05.
Results
The initial body weights and blood sugar levels of rats were close to each other. However at the end of the experiment in Group D and Group D + PII the average body weights were significantly decreased when compared to the control group (). In a similar way, in rats belonging to the diabetes groups (Group D, Group D + PI and Group D + PII) the blood sugar levels had increased significantly compared to the control group (, p < 0.05). However, kidney weights of all groups were similar to each other ().
Table 2. Body weight and blood glucose at beginning and end of the experiment.
Table 3. Kidney weight and apoptotic index of rats.
Histological findings
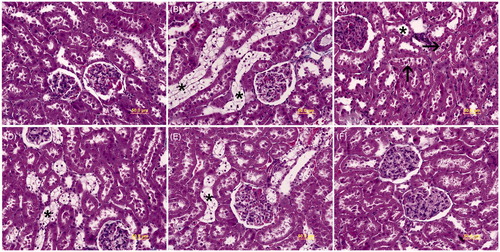
The microscopic examinations of kidney tissue belonging to the control () and Group P () subjects exhibited normal histomorphology. Dilatation in distal tubules (), desquamation of the epithelial cells of the tubules () and clear glycogen-filled distal tubules () were observed in Group D. Although, less often clear glycogen-filled distal tubules were also seen in Groups D + PI () and D + PII () kidney tissue subjects. However, in these groups, dilatation in distal tubules and epithelial desquamation into the lumen of tubules were not evident.
Figure 1. Light microscopy of kidney tissues is seen in different groups. (A) In controls, normal kidney architecture is observed. (B) Group D, transparent tubules (*) are detected. (C) Group D, dilatation in distal tubules (*) and epithelial desquamation into the lumen of tubules (arrow) are seen. (D) Group D + PI, transparent tubules (*) are exhibited. (E) Group D + PII, transparent tubules (*) are shown. (F) Group P, normal kidney architecture is observed. Kidney cross sections were stained with Masson’s Trichome.
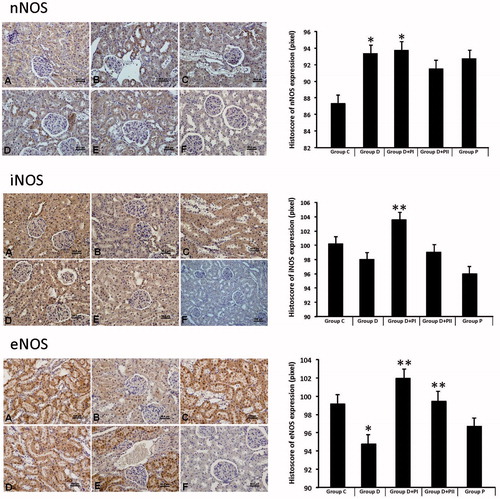
Immunohistochemistry findings
Immunohistochemical staining was performed using the avidin-biotin method to determine the kidney tissue expression of NOS. nNOS, iNOS and eNOS, the expression scoring results of the groups are shown in . Each of the three NOS (iNOS and eNOS, nNOS) expressions was especially observed in the corticomedullary region of the kidney tissue. Diabetic rats’ nNOS expression had increased and eNOS and iNOS expression had decreased compared to the control group. In Groups D + PI and D + PII, NOS expressions had improved compared to Group D. NOS expressions showed similarity in Group P to the control group.
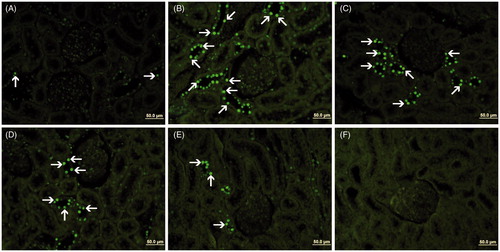
Apoptosis (TUNEL) findings
TUNEL staining was performed to determine apoptotic cells in kidney tissue (). The apoptotic index results are given in . The mean apoptotic index in the kidney of Group C and Group D was found to be 18.06 ± 2.64 and 30.30 ± 2.76, respectively. The increase in the apoptotic index was statistically significant in Group D compared to the Group C (p=0.002). Group D + PI and Group D + PII resulted in the decrease of the number of TUNEL- positive cells and the apoptotic index was 18.05 ± 1.90 and 13.00 ± 1.71, respectively. The decrease in the apoptotic index was statistically significant in Group D + PI (p=0.001) and Group D + PII (p=0.000) compared to Group D. The apoptotic index in the kidney of Group P was found to be 16.40 ± 1.85.
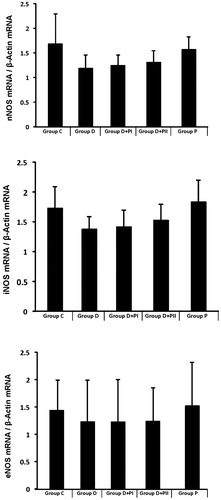
RT-PCR findings
In Group D, the nNOS, iNOS and eNOS mRNA levels decreased numerically compared to the control group. In the diabetes plus PTX groups, none of the three gene expression values changed ().
Discussion
Diabetes and diabetic kidney disease continue to increase worldwide. DN is the most common microvascular complication of DM and a major cause of ESRD. DN develops in about one-third of type 1 diabetic patients.Citation13 In DN, histopathologic changes such as thickening in glomerular and tubular basal membranes, glomerular and tubular hypertrophy, tubular vacuolation, mesangial cell proliferation and matrix increase were shown.Citation14 In the present study, in concordance with the literature, transparent tubules showing glycogen vacuolation and dilatation in the distal tubules with detachment and degeneration in the tubular epithelium were significantly observed in Group D. Also, the number of apoptotic cells increased in the Group D compared to Group C.
Numerous factors have been proposed for the pathogenesis of DN. Uncontrolled hyperglycemia, hyperlipidemia, systemic and intrarenal hypertension and activation of renin-angiotensin are the most important among these factors.Citation15 One of the mediators is NO. NOS is responsible for NO synthesis from l-arginine in mammalian cells. There are three isoforms of NOS: nNOS, iNOS, and eNOS. Changes in NOS enzymes in the diabetic kidney have been investigated in many studies. Yagihashi et al.Citation16 showed decreased immunoreactivity of nNOS in the macula densa and glomerular arterioles in diabetic rats. Similarly, in another study, it was shown that kidney nNOS activity decreased and protein levels were significantly lowered in STZ diabetic animals. However, insulin treatment failed to restore nNOS activity and protein expression.Citation17 In contrast, Shin et al.Citation18 and Komers et al.Citation19 reported an increase in nNOS production in STZ-induced diabetic rat kidney. Similarly, Yabuki et al.Citation20 reported an increase in immunoreactivity of nNOS in the kidneys of OLETF rats.
eNOS is thought to be involved in the pathogenesis of diabetic hyperfiltration. Clinical and experimental results suggest that renal eNOS expression and activity are upregulated in early diabetic kidneysCitation21; however, they are decreased with prolonged diabetes and the resulting vascular NO deficiency may facilitate the progression of DN.Citation22 For this upregulation or down regulation of eNOS, multiple factors and mechanisms have been proposed.Citation23 Therefore, it has been proposed that the eNOS could play a role in the development of DN.
High glucose levels decreased inducible NO production or availability in cultured mesangial cells.Citation24 Furthermore, some studies have shown reduced iNOS expressionCitation21 or unchanged iNOSCitation25 in diabetic kidneys. Considering the above studies, it is clear that the findings on NOS expression in the diabetic kidney are not compatible. In the present study, although nNOS mRNA was down regulated, increased immunoreactivity of cortical nNOS was exhibited. However, both protein and mRNA levels of eNOS and iNOS decreased in Group D.
Previous studies have shown that a variety of mechanisms are involved in DN pathophysiology and progression and that many of these share a common association with decreased renal NO production and increased ROS.Citation26 ROS is also closely linked to the production of RNS. Therefore; inflammatory molecules, oxidative stress and pathways are new potential targets for the treatment of DN. Different anti-inflammatory and antioxidants drugs have been offered as therapy for DN after many experimental studies performed in rodent models.Citation15
PTX is a methylxanthine phosphodiesterase inhibitor with antioxidant, anti-inflammatory effects and immune-regulatory properties.Citation27 Moreover, many studies have shown the beneficial effects of PTX in diabetic or non-diabetic kidney disease. It has been reported that PTX could provide an ameliorative effect against gentamicin,Citation9 cisplatin,Citation28 glycerolCitation29 and cyclosporine-induced nephrotoxicity.Citation30 In addition, PTX has shown beneficial effects in experimental models of DN and remnant kidney, with a reduction in renal hypertrophy, renal disease progression and urinary albumin excretion,Citation31 whereas clinical trials have demonstrated that PTF reduces clinical markers of glomerular and tubulo-interstitial injury in diabetic subjects.Citation32 In another study, Badri et al.Citation33 reported that PTX ameliorates microalbuminuria and proteinuria in patients with diabetic and non-diabetic kidney diseases. Garcia et al.Citation34 showed that PTX decreased blood glucose, fructosamine, HbA1c levels and immunoreactivity of iNOS, COX-2 and TNF-alpha in diabetic pancreas, liver and kidney. In addition, PTX (50 mg/kg) administered to rats for 8 weeks was shown to inhibit insulin resistance and prevent TNFalpha elevation, leukocyte infiltration and endothelial pyknosis in diabetic kidney.Citation35 Similarly, in another study, a decrease in HIF-1α and VEGF expression in the kidney of diabetic rats was shown.Citation36 However, the effect of PTX on NOS has not been investigated in the diabetic kidney so far. In the present study, we demonstrated that PTX ameliorated eNOS, iNOS and nNOS protein levels, but did not affect mRNA levels. Also PTX recovered the histological findings and number of apoptotic cells.
In conclusion, we showed that in chronic experimental DM, there is a reduction in eNOS and iNOS and an increase in nNOS. PTX decreases apoptotic cells and nNOS expression in diabetic rats. It also improves histological changes and immunoreactivity to iNOS and eNOS in the diabetic kidney. Thus, PTX has a healing effect on this damage by affecting NOS expression.
Disclosure statement
We declare that we have no conflict of interest.
References
- Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod Biomed Online. 2012;25:292–299.
- Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57:e1–526. A8,
- Turgut F, Bolton WK. Potential new therapeutic agents for diabetic kidney disease. Am J Kidney Dis. 2010;55:928–940.
- Sonmez MF, Narin F, Akkus D, Turkmen AB. Melatonin and vitamin C ameliorate alcohol-induced oxidative stress and eNOS expression in rat kidney. Ren Fail. 2012;34:480–486.
- Pollock JS, Pollock DM. Endothelin, nitric oxide, and reactive oxygen species in diabetic kidney disease. Contrib Nephrol. 2011;172:149–159.
- Honing ML, Morrison PJ, Banga JD, Stroes ES, Rabelink TJ. Nitric oxide availability in Diabetes Mellitus. Diabetes Metab Rev. 1998;14:241–249.
- Dellamea BS, Leitao CB, Friedman R, Canani LH. Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndr. 2014;6:17. DOI: 10.1186/1758-5996-6-17.
- Nasiri-Toosi Z, Dashti-Khavidaki S, Khalili H, Lessan-Pezeshki M. A review of the potential protective effects of pentoxifylline against drug-induced nephrotoxicity. Eur J Clin Pharmacol. 2013;69:1057–1073.
- Stojiljkovic N, Veljkovic S, Mihailovic D, et al. Protective effects of pentoxifylline treatment on gentamicin-induced nephrotoxicity in rats. Ren Fail. 2009;31:54–61.
- Navarro JF, Mora C, Muros M, Garcia J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: A short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16:2119–2126.
- Sonmez MF, Narin F, Akkus D, Ozdamar S. Effect of melatonin and vitamin C on expression of endothelial NOS in heart of chronic alcoholic rats. Toxicol Ind Health. 2009;25:385–393.
- Bayatli F, Akkus D, Kilic E, Saraymen R, Sonmez MF. The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: An experimental study. World J Urol. 2013;31:615–622.
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373.
- Donder E, Dogan MM, Kuloglu T, Dabak ÖD, Kocaman N, Ozkan Y. The investigation of the effects of enalapril and losartan on ghrelin immunoreactivity in kidney of streptozotocin-induced diabetic rats. Fırat Tıp Dergisi. 2013;18:1–6.
- Usuelli V, La Rocca E. Novel therapeutic approaches for diabetic nephropathy and retinopathy. Pharmacol Res. 2015;98:39--44.
- Yagihashi N, Nishida N, Seo HG, Taniguchi N, Yagihashi S. Expression of nitric oxide synthase in macula densa in streptozotocin diabetic rats. Diabetologia. 1996;39:793–799.
- Yu WJ, Juang SW, Chin WT, Chi TC, Chang CJ, Cheng JT. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci. 2000;68:625–634.
- Shin SJ, Lai FJ, Wen JD, et al. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologia. 2000;43:649–659.
- Komers R, Oyama TT, Chapman JG, Allison KM, Anderson S. Effects of systemic inhibition of neuronal nitric oxide synthase in diabetic rats. Hypertension. 2000;35:655–661.
- Yabuki A, Tahara T, Taniguchi K, Matsumoto M, Suzuki S. Neuronal nitric oxide synthase and cyclooxygenase-2 in diabetic nephropathy of type 2 diabetic OLETF rats. Exp Anim. 2006;55:17–25.
- Veelken R, Hilgers KF, Hartner A, Haas A, Bohmer KP, Sterzel RB. Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. J Am Soc Nephrol. 2000;11:71–79.
- Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007;18:2945–2952.
- Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286.
- Prabhakar SS. Tetrahydrobiopterin reverses the inhibition of nitric oxide by high glucose in cultured murine mesangial cells. Am J Physiol Renal Physiol. 2001;281:F179–F188.
- Schwartz D, Schwartz IF, Blantz RC. An analysis of renal nitric oxide contribution to hyperfiltration in diabetic rats. J Lab Clin Med. 2001;137:107–114.
- Lee EY, Lee MY, Hong SW, Chung CH, Hong SY. Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med J. 2007;48:847–855.
- Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ. Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev. 2012;2:CD006800.
- Kim YK, Choi TR, Kwon CH, Kim JH, Woo JS, Jung JS. Beneficial effect of pentoxifylline on cisplatin-induced acute renal failure in rabbits. Ren Fail. 2003;25:909–922.
- Akpolat T, Akpolat I, Ozturk H, et al. Effect of vitamin E and pentoxifylline on glycerol-induced acute renal failure. Nephron. 2000;84:243–247.
- Brunner LJ, Vadiei K, Iyer LV, Luke DR. Prevention of cyclosporine-induced nephrotoxicity with pentoxifylline. Ren Fail. 1989;11:97–104.
- Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–570.
- Navarro-Gonzalez JF, Muros M, Mora-Fernandez C, Herrera H, Meneses B, Garcia J. Pentoxifylline for renoprotection in diabetic nephropathy: The PREDIAN study. Rationale and basal results. J Diabetes Complications. 2011;25:314–319.
- Badri S, Dashti-Khavidaki S, Lessan-Pezeshki M, Abdollahi M. A review of the potential benefits of pentoxifylline in diabetic and non-diabetic proteinuria. J Pharm Pharm Sci. 2011;14:128–137.
- Garcia FA, Pinto SF, Cavalcante AF, et al. Pentoxifylline decreases glycemia levels and TNF-alpha, iNOS and COX-2 expressions in diabetic rat pancreas. Springerplus. 2014;3:283. DOI: 10.1186/2193-1801-3-283.
- El-Bassossy HM, El-Moselhy MA, Mahmoud MF. Pentoxifylline alleviates vascular impairment in insulin resistance via TNF-α inhibition. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:277–285.
- Sun HK, Lee YM, Han KH, Kim HS, Ahn SH, Han SY. Phosphodiesterase inhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J Intern Med. 2012;27:163–170.