Abstract
Purpose This study used the a nationwide population-based retrospective cohort study with the claims data from the Taiwan National Health Insurance Research Database to investigate the risk of urothelial carcinoma (UC) for hemodialysis (HD) patients. Methods The study population consisted of 2689 patients with end-stage renal disease (ESRD) newly diagnosed in 2000–2002 and underwent maintenance HD. Then, 21,449 reference patients were collected without HD randomly selected and matched with sex and age. The exclusion criteria were previous long-term analgesics and Chinese medication usage. Incidence density rates of UC in upper urinary tract (UTUC) and bladder (UBUC) were estimated for both cohorts by the end of 2012. Hazard ratios (HRs) of UC were measured in association with HD, covariates, and comorbidity. Results The incidence of UC was significantly higher in the HD cohort than in the reference cohort for both UT (21.8 vs. 0.65 per 10,000 person-years) and UB (17.7 vs. 3.55 per 10,000 person-years). The multivariate Cox proportional hazard regression analysis showed that the HRs of UTUC in HD cohort was 33.3 (95% CI = 15.9–69.5) and 5.14 for UBUC (95% CI = 3.24–8.15). The risk increased further for HD patients with comorbidity of hematuria, urinary tract infection (UTI) or hydronephrosis. Conclusion Patients with ESRD on HD are at a high risk of developing UC, especially UTUC in Taiwan. They will be paid more frequent to check urine analysis, urine cytology, and upper urinary tract survey.
Introduction
With an annual reported incidence of more than 3.31 per 10,000, the prevalence of end-stage renal disease (ESRD) in Taiwan is higher than in other countries.Citation1 Most patients with ESRD in Taiwan use hemodialysis, with only 9% using peritoneal dialysis and less than 1% receiving a kidney transplantation.Citation2 It also has been reported that urothelial carcinoma (UC) is the most common malignancy in Taiwanese patients with ESRD regardless of whether they undergo a kidney transplantation or dialysis.Citation3 According to the cancer registry of the Bureau of Health Promotion of Taiwan, 2050 patients had newly diagnosed urinary bladder urothelial carcinoma (UBUC) and 1174 had newly diagnosed upper urinary tract urothelial carcinoma (UTUC) in 2007 among the general population of 23 million people.Citation4 UBUC has been reported to account for 90–95% of cases of UC in Western countries, and UTUC only 5–10%.Citation5 Of note, UTUC accounts for a significantly higher proportion of cases of UC in Taiwan than in Western countries.Citation6
Chen et al reported that Taiwanese patients with ESRD are at a greater risk of UC.Citation7 In addition, Ou et al. reported a 10-fold higher incidence of UC among 1910 dialysis patients compared to the general population after an average follow-up period of 3.2 years.Citation8 However, the incidence of UTUC compared to UBUC in patients undergoing HD has yet to be clarified. In this study, we used representative claims data to conduct a retrospective cohort study comparing patients who were and were not undergoing HD and the subsequent risk of UC. We also investigated the symptoms and signs that were correlated with UC after HD.
Materials and methods
Data source
In this study, we used reimbursement data obtained from the Bureau of National Health Insurance (NHI), Department of Health, Taiwan. This universal insurance system was initialed in March 1995, with more than 96% of the whole population of 23 million people being enrolled by the end of 1996.Citation9 In this study, we used the reimbursement claims data of a randomly selected cohort of 1,000,000 people from the National Health Insurance Research Database (NHIRD). The distribution of gender and age of this cohort have been shown to be similar to the whole insured population in Taiwan. Data on gender, birth date, income, residential district or township, dates of diagnosis and receiving health care, and costs were available, and linked to scrambled identification numbers of the patients to ensure confidentiality.
Selection of HD and reference cohorts
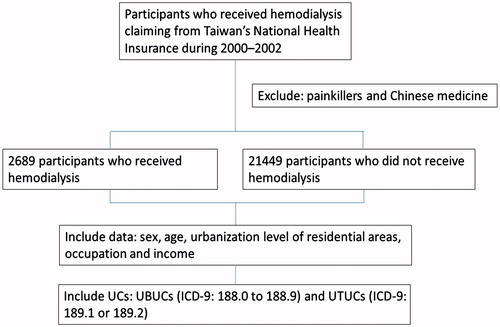
We searched the cohort for those who received health care based on International Classification of Disease, ninth Clinical Modification (ICD-9-CM) codes. Based on the insured population from 2000–2002, we identified 2689 patients who were 20 years of age or older with a new diagnosis of ESRD and who were receiving maintenance HD (ICD-9-CM code 39.95) as the HD cohort (). All potential reference candidates without HD were matched by age (every 10 years) of the HD cohort and the year when they started HD. Using a simple random selection method, we then identified 21,449 persons without HD as the reference cohort, all of whom were also 20 years of age or older. Patients with a diagnosis of cancer before entry to the study were excluded. We also excluded the patients who had visited Chinese medicine clinics and those with refillable prescriptions for painkillers, such as acetaminophen or nonsteroidal anti-inflammatory drugs, by screening their medical records.
Statistical analysis
We compared the distributions of sociodemographic factors including gender, age (20–59, 60–74, and ≥75 years), urbanization level of residential area, occupation (white collar, blue collar, and others) and income (<15,000, 15,000–24,999, ≥25,000 NT dollars) between the HD cohort and reference cohort without HD. We calculated the population density of each residential area. Areas with a population density in the lowest two quartiles were categorized as low urbanization areas, and those in the third and highest quartiles as moderate and high urbanization areas. White-collar workers were defined as those working at public institutions, schools, hospitals, businesses and the military and blue-collar workers were defined as those working at fishers, farm workers, and industrial workers. All other subjects were defined as being retired or unemployed. We used the chi-square test to assess the distributions of these variables between cohorts.
UBUC (ICD-9-CM codes 188.0 to 188.9) and UTUC (ICD-9-CM codes 189.1 or 189.2), including those at the renal pelvis and ureter, were identified in both cohorts, who were followed-up until 2012. The incidence density of UC in each cohort and HD to reference cohort rate ratios were calculated using the sociodemographic variables. The age of the subjects was defined as that at entry into the study. Follow-up person-years were calculated according to the variable of interest until the date of the first diagnosis of UC, death, withdrawal from the insurance program, or the last date of follow-up. Cox proportional hazard analysis was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) of UC for the HD cohort compared with the reference cohort, controlling for the independent effects of sociodemographic factors and comorbidities. We excluded those using analgesics, such as Ponstan (mefenamic acid) by screening for the Taiwan health insurance drug coding AB08774100 (PONSTAN F.C. TABLETS) and NB01043100 (PONSTAN KAPSEALS). Patients were excluded if they had visited or used Chinese medicine. We also considered the concurrent clinical presentation of hematuria (ICD-9-CM code 599.7), urinary tract infection (UTI) (ICD-9-CM 599.0 and A-code A3595), and hydronephrosis (ICD-9-CM code 591 and A-code A3559) in the HR estimation. All statistical analyzes were performed using the SAS statistical package (version 9.1; SAS Institute, Cary, NC). The level of statistical significant was defined as a p values < 0.05.
Results
There was no significant difference in gender between the HD and reference cohorts (). Compared with the reference cohort, the HD cohort was significantly older, had fewer white-collar workers (both p < 0.001), was more likely to live in a less urbanized area (p = 0.003) or in areas other than northern Taiwan (p < 0.001), and had a lower income (p < 0.001).
Table 1. Comparisons in sociodemographic factors between cohorts with and without hemodialysis.
Of the 2689 patients on HD, 67 (2.49%) subsequently developed UC, 37 (55.2%) with UTUC and 30 (44.8%) with UBUC (). Among 58 (0.27%) cases of UC in the reference cohort, nine (15.5%) had UTUC, and 49 (84.5%) had UBUC. The overall incidence density of UTUC was 33.4 times greater in the HD cohort than in the reference cohort (21.8 per 10,000 person-years vs. 0.65 per 10,000 person-years). Women were at a higher risk of developing UTUC (44.3 vs. 25.1 per 10,000 person-years) than men. The HD patients who were younger, blue-collar workers, living in a low urbanized or southern area, and with a moderate income had higher incidence rates of UTUC.
Table 2. Incidence density of UTUCs stratified by sociodemographic status in hemodialysis cohort and nonhemodialysis cohort.
In comparison, the incidence density of UBUC was 5.0 times higher in the HD cohort than in the reference cohort (17.7 vs. 3.56 per 10,000 person-years) (). The incidence rates were higher in women, those who were younger, and those living in southern areas. The rate ratios showed that the HD patients who were blue collar workers and those with a higher income had a higher risk of UBUC.
Table 3. Incidence density of UBUCs stratified by sociodemographic status in hemodialysis cohort and non-hemodialysis cohort.
shows the results of multivariate Cox proportional hazard regression analysis for the risk of developing UTUC and UBUC. Compared with the reference cohort, the patients in the HD cohort were at a higher overall risk of UC, including 33-fold for UTUC (95% CI = 15.9–69.5, p < 0.001) and about 5-fold for UBUC (95% CI = 3.24–8.15, p < 0.001). Compared with men undergoing HD, women undergoing HD had a higher risk of UC. This risk was significant higher for UBUC with an excess hazard of 82% (95% CI = 14–191%) but not significantly higher for UTUC (HR = 1.24; 95% CI = 0.69–2.24).
Table 4. Hazard ratios and 95% confidence intervals of developing UTUCs and UBUCs associated with hemodialysis controlling for sociodemographic factors.
Patients undergoing HD who developed UC were significantly more likely to have the comorbidities of hematuria (40.3% vs. 13.9%, p < 0.001), UTI (61.3% vs. 31.3%, p < 0.001), and hydronephrosis (19.4% vs. 8.5%, p = 0.002) than those without UC. We further investigated whether there were any associations between these factors and HD in the development of UC. Cox proportional hazard regression analysis () revealed significant associations between hematuria (HR = 87.0, 95% CI = 48.1–157 for UTUC; HR = 16.8, 95% CI = 9.8–28.7 for UBUC), UTI (HR = 11.0, 95% CI = 6.10–19.9 for UTUC; HR = 3.54, 95% CI = 2.03–6.16 for UBUC) and hydronephrosis (HR = 38.7, 95% CI = 20.2–74.0 for UTUC; HR = 11.0, 95% CI = 5.07–24.0 for UBUC), after controlling for gender and age.
Table 5. Hazard ratios of UCs in hemodialysis patients with comorbid hematuria, UTI, and hydronephrosis controlling for sociodemographic status.
Discussion
Generally speaking, UTUC is relatively rare and accounts for only 2% to 5% of urothelial tumors, with renal pelvis lesions representing the majority of cases.Citation9 The cancer registry in Taiwan shows that the incidence rates of UTUC (n = 1174, or 1.5% of all cancers) diagnosed in 2007 were 4.88 per 1,000,000 in males and 5.35 per 1,000,000 in females. In comparison, among 2050 patients with newly diagnosed UBUC (2.7% of all cancers), the incidence was more than 2-fold higher in males than in females (12.55 per 1,000,000 vs. 5.22 per 1,000,000).Citation10 We found female are more prone to develop UTUCs in the uremic cohort. Some studies were reported that there are many possibilities such as urinary TB infection, gene, or carcinogenicity that may explain the increased ratio of female patients in contrast to the male predominance.Citation11,12 Furthermore, there are many studies in Chinese population show that unusual increase in the ratio of female-to-male incidence in UTUC which accounts for approximately 30–40% of all cases of UC in Taiwan, which is different from other countries.Citation13,14
The incidence rate of ESRD in Taiwan increased from 375 per 1,000,000 in 2004 to 404 per 1,000,000 in 2005,Citation15 and the prevalence of ESRD in Taiwan is the highest worldwide.Citation16 In a hospital-based study in Taiwan, Ou et al. reported that the risk of UC was 10-fold higher in dialysis patients than in those without dialysis (0.99% vs. 0.10%).Citation8 Chiang et al. also reported a similar incidence of primary UTUC (0.93%) in dialysis patients.Citation16 However, we found a higher risk of UTUC and/or UBUC in patients with ESRD undergoing maintenance HD, of whom 2.49% developed UC. Other studies have also reported a higher incidence of concomitant UC in patients with chronic renal failure undergoing maintenance dialysis or a kidney transplantation in Taiwan.Citation17 In addition, the time to the development of UTUC compared to renal cell carcinoma in dialysis patients has been reported to be much shorter in Taiwan than in Japan (54 vs. 143 months).Citation18
Of the 67 cases of UC in the patients undergoing HD in this study, 37 (55.2%) were diagnosed in the renal pelvis or ureters, and 30 (44.8%) in the bladder. This suggests that HD may be a risk factor for UTUC and is similar to the study by Chang et al., in which 54% of cases of UC involved the upper urinary tract in HD patients.Citation19 Wu et al. also reported a higher recurrence rate of UTUC in patients on dialysis than in those not on dialysis in Taiwan. They also emphasized the risk of UTUC in patients with uremia.Citation20
With regards to the etiology of UTUC, the reported risk factors include the use of herbal remedies (aristolochic acid; AA), analgesics (phenacetin and nonsteroidal anti-inflammatory drugs), heavy metals (arsenic), and tobacco smoking.Citation21 AA is thought to be the underlying cause of Chinese herbal nephropathy, which is a rapidly progressive interstitial fibrotic renal disease that frequently results in urothelial malignancies. AA has also been demonstrated to be a urothelial carcinogen and to be toxic to the kidneys, which may also contribute to the development of ESRD.Citation22 Jensen et al. reported that UTUC and UBUC share a common histology and similar risk factors, such as AA.Citation23 The use of AA and related products has now been banned in Taiwan for more than 15 years.Citation24
The long-term high-dose use of analgesics, such as of phenacetin and nonsteroidal anti-inflammatory drugs, may result in analgesic nephropathy and a 20-fold increased risk of UTUC.Citation25 Some studies have reported that an increased risk of UC in patients with ESRD is associated with the usage of Chinese herbal products and analgesics, especially in the upper urinary tract.Citation26 A preliminary study investigating the effect of arsenic in renal parenchymal tissue of patients with UC and ESRD did not find any increase in the metal compared to patients without UC. Analgesics are prohibited in patients with chronic renal disease in Taiwan.
Besides age and gender, smoking is also considered to be an important risk factor for UC.Citation27 Smoking cigarettes and exposure to secondhand smoke have been shown to be significant risk factors for developing UC in Taiwan; however, the use of tobacco in Taiwan has been reported to be lower than in other countries at about 40% in males and 5% or lower in females.Citation28 All patients with ESRD are advised to avoid smoking in Taiwan; however, one national retrospective study found that the time to the development of UTUC was not related to cigarette consumption.Citation24 Taken together, AA, arsenic, analgesics, and smoking do not seem to explain the higher trend of UTUC in Taiwan.
In this study, we investigated patients with uremia undergoing maintenance HD but not those who received a kidney transplantation or peritoneal dialysis because surgery and post-transplantation immunosuppressant agent treatment increase the risk of malignancy. It has been suggested that the urine of patients with ESRD contains toxins that may cause carcinomas, such as UC, and that this may explain the higher risk of UC in patients with ESRD than in those with normal renal function. Furthermore, patients undergoing HD would have a reduced urine flow from the urinary tract to the urinary bladder. Maisonneuve et al. reported that the risk of UC in patients undergoing maintenance HD was higher in the first year, and especially in Australia and New Zealand.Citation29 Therefore, progressive exposure to toxins may occur in the urinary tract, and this may explain the higher incidence of UTUC than UBUC in HD patients.Citation30
The other important finding of this study is that HD patients with the comorbidities of hematuria, urinary tract infection or hydronephrosis had a higher risk of UC, especially in the upper urinary tract. Wu et al. reported that patients on dialysis with painless gross hematuria or bloody urethral discharge had an extremely high risk of developing UC.Citation31 In addition, Chang et al. concluded that gross hematuria should never be ignored in patients with ESRD.Citation19 Such symptoms and signs are considered to be good indicators for further detailed examinations of the urinary tract, such as cystourethroscopy, with retrograde pyelography to rule out concurrent UC.
There are some limitations to this study. First, it is a retrospective study with a small sample size. However, we used representative data from a nationwide database, and there is little likelihood of selection or information bias. Second, the NHIRD lacks some important clinical data, such as tumor grading, family history, and the frequency of hemodialysis. However, an advantage of our study is that we excluded risk factors such as the use of Chinese medicinal herbs and analgesics to minimize bias. Third, the number of patients with uremia and UC may have been underestimated because the data in the NHIRD came from contracted NHI practitioners and excluded non-NHI data (including self-paying patents). Fourth, we did not know the causes of ESRD in the HD group, and we did not exclude comorbidities of hypertension or diabetes mellitus. Nevertheless, we tried to minimize bias by comparing age-matched groups. In addition, we did not elucidate the factors resulting in the high incidence of UTUC, especially in the uremic patients on hemodialysis. However, we did demonstrate that a strong association between UC and uremia was much more common in the HD patients with UTUC than in those with UBUC. Comparisons of sociodemographic factors between the cohorts with and without HD showed that the patients undergoing HD tended to be blue-collar workers, living in less urbanized areas with a lower income. Further studies are needed to investigate other possible risk factors for UTUC. We included 2689 patients over a 10-year follow-up period after starting dialysis. In addition, this study is a multicenter collaborative study that makes the findings more credible.
Conclusions
Patients with uremia undergoing HD were associated with an increased risk of developing UC and particularly UTUC.Citation11 Hematuria, UTI or hydronephrosis were risk factors for developing UC among the HD patients. Physicians should be alert to HD patients presenting with hematuria, dysuria or flank discomfort by regularly performing urine analysis and upper urinary tract surveys (renal echo, retrograde pyelography, magnetic resonance imaging, and computed tomography urography) because of the potential risk of UC.Citation32 Those at risk should also avoid the use of AA, analgesics, and smoking. Further studies on HD may confirm the etiology of UTUC in Taiwan.
The authors thank the National Health Research Institute in Taiwan for providing us with insurance claims data.
Funding information
This study was supported partly by the Department of Health, Executive Yuan, Taiwan, Republic of China (grant number DOH 97-HP-1101, 2008–2010), the Clinical Trial and Research Center for Excellence (grant number DOH100-TD-B-111–004) the Cancer Research Center of Excellence (DOH100-TD-C-111–005), and China Medical University Hospital (grant number 1MS1).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hwang SJ, Yang WC, Hwang SC, Lin HM. Data analysis from the applications for uremic major illness card from 2001 to 2003. Acta Nephrologica. 2005;19:376.
- 2nd Congress of the Asian Transplantation Society. Proceedings. Taipei, Taiwan, November 26–28, 1991. Transplant Proc. 1992;24:1259–1643.
- Jiaan BP, Yu CC, Lee YH, Huang JK. Uraemia with concomitant urothelial cancer. Br J Urol. 1993;72:458–461.
- Cancer Registry annual report, Bureau of health promotion department of health, the Excusive Yuan, Taiwan. The incidence of bladder, renal pelvic and ureteral tumor in Taiwan. Available at: https://cris.bhp.doh.gov.tw/pagepub/Home.aspx?itemNo=cr.q.10. Accessed March 15, 2011.
- Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J Urol. 2000;164:1523–1525.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet].
- Chen KS, Lai MK, Huang CC, Chu SH, Leu ML. Urologic cancers in uremic patients. Am J Kidney Dis. 1995;25:694–700.
- Ou JH, Pan CC, Lin JS, et al. Transitional cell carcinoma in dialysis patients. Eur Urol. 2000;37:90–94.
- Milojevic B, Dzamic Z, Kajmakovic B, Milenkovic Petronic D, Sipetic Grujicic S. Urothelial carcinoma: Recurrence and risk factors. J Buon. 2015;20:391–398.
- The incidence of bladder, renal pelvic and ureteral tumor in Taiwan. Available at: https://cris.bhp.doh.gov.tw/pagepub/Home.aspx?itemNo=cr.q.10. Accessed March 15, 2011.
- Chou YH, Huang CH. Unusual clinical presentation of upper urothelial carcinoma in Taiwan. Cancer. 1999;85:1342–1344.
- Lien YC, Wang JY, Lee MC, et al. Urinary tuberculosis is associated with the development of urothelial carcinoma but not renal cell carcinoma: A nationwide cohort study in Taiwan. Br J Cancer. 2013;109:2933–2940.
- Wang Y, Lang MR, Pin CL, Izawa JI. Comparison of the clonality of urothelial carcinoma developing in the upper urinary tract and those developing in the bladder. Springer Plus. 2013;2:412.
- Tang Q, Xiong G, Li X, et al. The prognostic impact of squamous and glandular differentiation for upper tract urothelial carcinoma patients after radical nephroureterectomy. World J Urol. 2015. [Epub ahead of print]. DOI: 10.1371/journal.pone.0129268.
- Taiwan Society of Nephrology. The Ministry of Health and Welfare of the Republic of China. Available at: http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=1029&webdata_id=2122. Accessed January 27, 2009.
- Kang CH, Chen CH, Chiang PH. Primary urothelial carcinoma of the upper urinary tract in dialysis patients with 5-year follow-up. Jpn J Clin Oncol. 2010;40:241–246.
- Wu MJ, Lian JD, Yang CR, et al. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan. Am J Kidney Dis. 2004;43:1091–1097.
- Kao YL, Ou YC, Yang CR, Ho HC, Su CK, Shu KH. Transitional cell carcinoma in renal transplant recipients. World J Surg. 2003;27:912–916.
- Chang CH, Yang CM, Yang AH. Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer. 2007;109:1487–1492.
- Wu CF, Shee JJ, Ho DR, Chen WC, Chen CS. Different treatment strategies for end stage renal disease in patients with transitional cell carcinoma. J Urol. 2004;171:126–129.
- Messing EM, Catalona W. Urothelial tumors of the urinary tract. In: Walsh PC, Retik AB, Vaughan ED and Wein AJ, Eds., Campbell’s Urology. Philadelphia: WB Saunders; 1998;2327–2410.
- Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686–1692.
- Jensen OM, Knudsen JB, McLaughlin JK, Sorensen BL. The Copenhagen case-control study of renal pelvis and ureter cancer: Role of smoking and occupational exposures. Int J Cancer. 1988;41:557–561.
- Wang SM, Lai MN, Chen PC, et al. Increased upper and lower tract urothelial carcinoma in patients with end-stage renal disease: A nationwide cohort study in Taiwan during 1997–2008. BioMed Res Int. 2014;2014:149750.
- Palvio DH, Andersen JC, Falk E. Transitional cell tumors of the renal pelvis and ureter associated with capillarosclerosis indicating analgesic abuse. Cancer. 1987;59:972–976.
- Wang SM, Lai MN, Wei A, et al. Increased risk of urinary tract cancer in ESRD patients associated with usage of Chinese herbal products suspected of containing aristolochic acid. PLoS One. 2014;9:e105218.
- Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta-analysis of epidemiologic studies. Cancer. 2000;89:630–639.
- Wu CC, Chen MC, Huang YK, et al. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J Formos Med Assoc. 2013;112:554–560.
- Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet. 1999;354:93–99.
- Heidland A, Bahner U, Vamvakas S. Incidence and spectrum of dialysis-associated cancer in three continents. Am J Kidney Dis. 2000;35:347–351. discussion 352–353.
- Wu CF, Chang PL, Chen CS, Chuang CK, Weng HH, Pang ST. The outcome of patients on dialysis with upper urinary tract transitional cell carcinoma. J Urol. 2006;176:477–481.
- Wang LJ, Lee SY, Teh BT, Chuang CK, Nortier J. Upper tract urothelial carcinomas in patients with chronic kidney disease: relationship with diagnostic challenge. BioMed Res Int. 2014;2014:989458.