Abstract
Amikacin (AK) is frequently used on the treatment of Gram-negative infections on neonates, but its usage is restricted because of nephrotoxicity. In this study, on neonatal rats, we aimed to investigate the effects of erythropoietin and vitamin E on AK induced nephrotoxicity. A total of 35 newborn Wistar Albino rats were divided into four groups: (1) injected with saline (serum physiological was administered to placebo controls), (2) injected with AK (1200 mg/kg), (3) injected with AK + vitamin E (150 mg/kg), (4) injected with AK + erythropoietin (EPO) (300 IU/kg/day). In renal tissue, AK levels were significantly high in all groups except the control. Tissue malondialdehyde (MDA) and nitric oxide (NO) levels were statistically higher in AK -treated group than the control. MDA and NO levels were significantly decreased with the administration of vitamin E and EPO. Glutathione peroxidase (GPX) levels were statistically low in AK group compared with the controls. The levels of GPX, in vitamin E group, were increased significantly. However, superoxide dismutase and catalase levels were not significantly different in none of the groups. Insulin-like growth factor-1 values in AK, EPO and vitamin E groups were significantly higher than the control group. Histomorphological changes such as tubular epithelial necrosis were seen in AK treated group. Histopathological improvements observed with EPO and vitamin E administration. AK nephrotoxicity is related to oxidative stress and is supported with biochemical and histopathological findings. Vitamin E and EPO, as antioxidants, can be useful renoprotective agents for ameliorating AK induced nephrotoxicity in neonates.
Introduction
Aminoglycoside antibiotics, especially amikacin (AK), are widely used in neonatal intensive care units for the treatment of severe, life-threatening Gram-negative bacterial infections. AK, which is frequently used on the treatment of Gram-negative infections on neonates, has high antibacterial efficacy, rapid onset of action, synergy with beta-lactam antibiotics, low resistance and low costs. Despite all its benefits, the clinical usage is restricted because of nephrotoxicity. Renal dysfunction mechanism of AK is not well known. The nephrotoxic side effects of AK have been documented in numerous species of studies.Citation1–8 Experimental studies had suggested that free oxygen radicals play a role in the pathogenesis of nephropathy and it has also been shown that toxicity might be prevented with several antioxidants.Citation9–13
Both vitamin E and erythropoietin (EPO) frequently use in neonatal intensive care units for supplementation. It has been shown that dietary supplementation of vitamin E suppresses oxidative stress and glomerulosclerosis in rat kidney.Citation14–16 Hematopoietic growth factor, EPO, is widely used in the treatment of anemia various of clinical conditions. Recombinant human EPO (rhEPO) has been shown to stimulate endothelial cell proliferation and angiogenesis.Citation17,Citation18 EPO and its receptor are expressed in multiple tissues, such as in glomerular, mesangial and tubular epithelial cells in human, rat and mouse kidney.Citation19,Citation20 The antiapoptotic effects of EPO have been shown in different models of nephrotoxicity but there is not enough evidence regarding the antioxidant effects of EPO on renal injury induced by AK.Citation21–23
In this study, we aimed to investigate the role of oxidative tubular damage on AK induced nephrotoxicity and to evaluate the effects of antioxidants on biochemical and histopathological changes on neonatal rats.
Materials and methods
Animal protocol
The present study was performed in accordance with the guidelines provided by the Experimental Animal Laboratory and approved by the Animal Care and Use Committee of our institution. Thirty-five healthy newborn Wistar albino rats (age 3–7 days, birth weight 4–7 g) were used. Rats were kept with their mothers, fed with breast milk, in a temperature-controlled (22–24 °C) environment, with 55–60% humidity under a 12-h light–dark cycle for 7 days through experimentation.
Experimental protocol
A preliminary experiment was performed to determine the appropriate dosage to make nephrotoxicity to the newborn rats. After determining doses, rats were randomly allocated into four groups as follows: (i) Group 1, control (n = 8); (ii) Group 2, AK treated (n = 8); (iii) Group 3, AK + Vitamin E treated (n = 9) and Group 4, AK + EPO treated (n = 10). Group 1 injected with saline intraperitoneally (i.p.) for 3 days (4th, 5th, 6th days). AK (Amikozit flacon 0.5 g/2 mL; Eczacıbaşı, Istanbul, Turkey) was dissolved in distilled water and administered to Groups 2, 3 and 4 with a single dose of 1200 mg/kg, i.p., for 3 days. Vitamin E (Evigen ampul 300 IU/2 mL; Aksu-Farma, Istanbul, Turkey) was given at a dose of 150 mg/kg/day, i.p. 30 min before AK administration for 3 days.Citation24 Recombinant human EPOa (Eprex; 2000 UI/mL; Santa-Farma-Gurel, Istanbul, Turkey) was given at a dose of 300 UI/kg/day, i.p. 30 min before AK administration for 3 days.Citation23
The daily dose of AK was administered 1/2 h after vitamin E and EPO treatment in the morning (i.e., vitamin E and EPO was given at 08:30 h and AK was given at 09:00 h). Each injection was administered at the same time of the day. Isotonic saline solution (an equal volume to AK) was administered to Group 1 by i.p. injection. In addition, distilled water (an equal volume to vitamin E/EPO) was given i.p. to Groups 1 and 2.
Specimen collection and methods
Rats were anesthetized with ether and killed 24 h after the last injection. Both kidneys were removed, weighed, decapsulated and divided equally into two longitudinal pieces. One-half of the right kidney was placed in formaldehyde solution for routine histopathological examination by light microscopy. The entire left kidney and the other half of the right kidney were washed with physiological saline for analysis of malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), nitric oxide (NO), glutathione peroxidase (GPX) and insulin-like growth factor (IGF-1) and determination of tissue concentrations of AK. Tissue samples were suspended in 3 mL Tris–HCl buffer, pH 7.3, that contained 0.25 mol/L sucrose and were stored at −80 °C until further analysis.
Renal tissue was homogenized in a motordriven tissue homogenizer (IKA Ultra-Turrax T25 Basic; Labortechnic, Staufen, Germany) with phosphate buffer (pH 7.4). Unbroken cells, cell debris and nuclei were sedimented by centrifugation at 5000g for 10 min at +4 °C. After homogenization, biochemical analysis was performed immediately.
Renal AK accumulation in kidney tissue supernatant was measured using commercially available kits by fluorescence polarization immunoassay by using Aoroset automatic analyzer by standard spectrophotometric methods (Max Planck-Ring, Wiesbaden, Germany).
Tissue MDA levels were determined from the homogenate by the double heating method of Draper and Hadley.Citation25 The principle of this method is the spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid (TBA) with MDA. The prepared solution was cooled under tap water and its absorbance was measured using a spectrophotometer (Shimadzu UV-1601; Shimadzu, Kyoto, Japan) at 532 nm. The concentration of MDA was calculated by the absorbance coefficient of the MDA–TBA complex (absorbance coefficient = 1.56 × 105 L/mol/ cm) and is expressed as nmol/g protein in the kidney.
NO was assayed colorimetrically using the Griess reaction (Sigma Co., St. Louis, MO). The Griess reagent reacted with nitrite to form a pink to dark pink color after incubation for 10 min. The absorbance of the samples was read at 540 nm and levels, expressed as micromolar of protein, were determined using a standard NaNO2 solution.
SOD activity was estimated in the supernatant according to the method described by Sun et al.Citation26 The measurement of SOD activity is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine/xanthine oxidase system as a superoxide generator. Activity was assessed in the ethanol phase of the supernatant after 1.0 mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged at 15,000g for 3 min. One unit of SOD activity was defined as the amount of enzyme causing 50% inhibition of reduction of NBT reduction. Activity is expressed as U/g protein.
GPX activity was determined spectrophotometrically at 340 nm by the method of Paglia and Valentine.Citation27
CAT activity was measured using the method described by Aebi.Citation28 The principle of the assay is based on the determination of the rate constant k (s−1) of hydrogen peroxide decomposition. By measuring the change in absorbance per minute, the rate constant for the enzyme was determined. Activity is expressed as k (rate constant)/g protein.
Protein levels in the homogenate and supernatant were determined according to the method of Lowry et al.Citation29
IGF-I concentrations were determined by a hydrochloride acid–ethanol extraction radioimmunoassay (RIA), using human IGF-I for labeling (Nichols Institute Diagnostics, San Juan Capistrano, CA) according to the method of Sjöberg et al.Citation30
Histolopathological evaluation
Half of the right kidneys of the rats were fixed in 10% buffered formalin and then placed in fresh fixative solution and embedded in paraffin, sectioned at 5 μm and stained with hematoxylin–eosin. A pathologist, blinded to sample identity, examined all samples by using light microscopy. Light microscopy (Olympus BH-2; Olympus, Tokyo, Japan) was used to semiquantitatively evaluate kidney sections ().
Figure 1. Control: Photomicrograph of the cortex of a kidney from Group 1 (control) showing normal renal histology (HE, 40×).
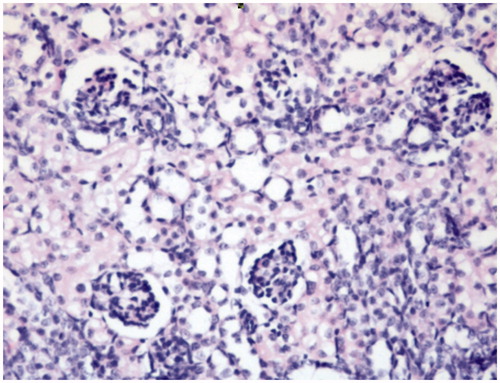
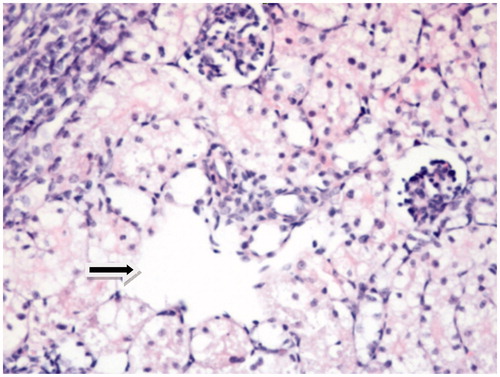
Figure 3. AK + vitamin E: Dilatation of tubules, vacuolization in the proximal tubules and the normal structure of glomeruli are seen. There is no inflammation in the interstitial space (HE, 40×).
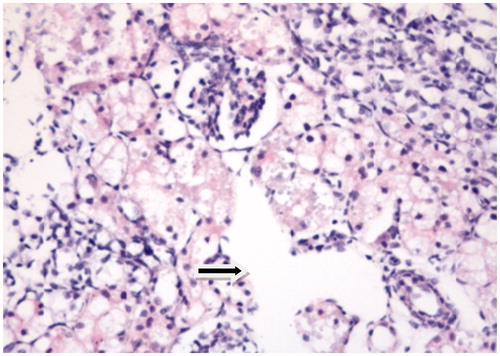
Tissues were examined for tubular epithelial alterations (dilatation, desquamation, vacuolization, necrosis, atrophy, casts), interstitial inflammatory cell infiltration, edema and glomerular changes. All histopathological parameters were graded as followsCitation23:
Level – (normal) = no changes.
Level + (mild) = single cell necrosis, slight degenerative changes, few foci of dilatation, casts, inflammatory infiltration and edema.
Level ++ (moderate) = changes at different foci throughout the kidney.
Level +++ (severe) = severe damage or necrosis.
Statistical analysis
The data were analyzed using statistical package for social sciences (SPSS) 11.0 software program (SPSS, Chicago, IL). One-way analysis of variance and post hoc multiple comparison tests (least-squares difference) were performed on biochemical variables to examine differences among the four groups. All data are expressed as the mean ± standard deviation and p values of <0.05 were considered statistically significant.
Results
In renal tissue, AK levels were significantly high in all groups except the control group. AK induced acute renal toxicity, manifested by a significant increase in kidney lipid peroxides measured as MDA and NO (). Tissue MDA and NO levels were statistically higher (p < 0.05) in AK-treated group than the control. MDA and NO levels were decreased with the administration of vitamin E and EPO and the difference was statistically significant.
Table 1. Biochemical data in the four experimental groups (mean ± SD).
GPX levels were statistically low in AK group compared with the controls. The levels of GPX, in vitamin E group, were increased significantly. However, SOD and CAT levels were not significantly different in none of the groups.
IGF-1 values in AK, EPO and vitamin E groups were significantly higher than the control group.
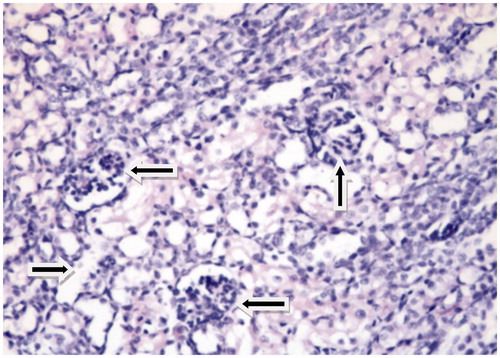
The grading of histological changes is summarized in . By histopathological investigations, clear histomorphological changes such as tubular epithelial necrosis, tubular dilatation, vacuolar degeneration and edema were seen in AK treated group. Histopathological improvements observed with EPO administration were evident, but imperceptible with vitamin E.
Table 2. Histopathological changes in renal tissues.
Discussion
Aminoglycosides appear to generate their nephrotoxic side effects by three general mechanisms: renal tubular toxicity, reduced glomerular filtration and reduction in renal blood flowCitation31. AK is an aminoglycoside antibiotic, which is used in clinical practice to treat severe Gram-negative infections. However, its nephrotoxicity has limited the extend use of it. Proximal renal tubular cells are the primer site of damage in patients treated with the antibiotic AK. The nephrotoxic side effects of aminoglycoside antibiotics have been documented in numerous species of experimental animalsCitation2,Citation32. Over the production of free radicals induces the lipid peroxidation (MDA), by destroying unsaturated fatty acids in the cell membrane.
Other scientists have used different methods of assessment of renal damage in an attempt to determine the relative sensitivity of the kidney to AK as well as the effect of age and doseCitation3–7,Citation10,Citation33. One mechanism of this toxicity is believed to involve reactive oxygen radical species generation (ROS). Also, it has been shown that aminoglycoside antibiotics exert their adverse renal effects by generating of ROS. Some studies demonstrated that antioxidant administration has ameliorated AK induced nephrotoxicityCitation9,Citation11,Citation12. The aim of the present study was to evaluate the effects of vitamin E and EPO on antioxidative state of AK induced nephrotoxicity in neonatal rats. Before starting the study, a preliminary experiment was performed to determine the appropriate dosage to make nephrotoxicity to the newborn rats.
In this study, AK administration to rats increased MDA levels and vitamin E and EPO administration significantly decreased MDA production in the AK rats’ kidneys compared with the only AK treated rats. In the present study, we created that marked nephrotoxicity by intraperitoneal administration of AK were high levels of AK and marked histological changes in the renal tissue were observed in Groups 2–4 rats. It is also evaluated AK induced renal damage and investigated the protective role of vitamin E and EPO using biochemical and oxidative markers (MDA, NO, SOD, GPX, CAT, IGF-1) and the morphology of the kidney as examined under light microscopy, commonly used to monitor the development of renal damage. The histopathological examination of kidneys revealed that there was a significant tubular damage indicated by tubular dilatation, tubular vacuolization and interstitial edema. Also, histopathological findings were parallel with the biochemical findings in AK treated group and this shows that free radicals may have critical role in renal nephrotoxicity and EPO or vitamin E is protective via their antioxidant properties.
In the kidney, the proximal tubule is responsible for the excretory transport from blood to urine of xenobiotics, xenobiotic metabolites and waste products of metabolismCitation34. Aminoglycosides are known to be nephrotoxic and make their endothelial injury by reducing the structure and density of fenestrated endotheliumCitation1. NO is the part of endothelin-signaling cascade in the kidney and is produced by NO synthase (NOS). Both NOS and endothelin were found to be localized to the same nephron segments, including the proximal tubuleCitation35,Citation36. Studies emphasize the increase of MDA and NO levels, which are the end product of lipid peroxidation in oxidative stress damageCitation11,Citation12. In literature, NO has been implicated in cyclosporin A induced nephrotoxicity in renal proximal tubulesCitation37. Another study indicates that, when proximal tubules were exposed to radiocontrast agents, aminoglycoside antibiotics or heavy-metal salts, NO production increasedCitation34. NO production has been implicated in renal diseases and the action of several nephrotoxic compoundsCitation38. In this study, renal tissue MDA and NO levels were statistically high in AK treated group. Another study has shown that plasma NO levels and the nephrotoxivity was statistically high in gentamicin treated ratsCitation39.
Although CAT and GPX using the same substrate for their activities, in this study, a significant difference between the groups for GPX activity was found but CAT activity was not affected. GPX is the first step enzyme defense against H2O2; subsequently, CAT takes part in enzymatic reaction. It could be explained by the short time of the study. This study made on newborn rats and the tissue samples were taken within 3 days. We thought that if the time was much longer we could see the decline of CAT activity.
EPO acts by binding to its specific receptor on the surface of erythroid progenitor cells to stimulate cell survival, proliferation and differentiationCitation40. In animal models, EPO treatment in rodents was also observed to stimulate NO productionCitation41–43. EPO is the primary erythroid cytokine that provides for up-regulation of mature red blood cell production in response to hypoxic or ischemic stressCitation44.
In the study, renal tissue MDA and NO levels were statistically high in AK treated group and these levels were significantly decreased with the administration of EPO. Similarly, Kaynar et al.Citation45 emphasize the role of EPO in the prevention of AK induced nephropathy and they found that serum urea levels were significantly improved in rats treated with EPO. In this study, we could not able to take serum specimen for urea and creatinine because our study groups were newborn rats and when we finish the study the rats were only 7 g.
Several studies have shown that rhEPO may play an important role also in the prevention of cisplatin-induced nephrotoxicityCitation22,Citation46. Renoprotective effect of rhEPO also evaluated in vancomicine induced nephrotoxicity in rats and it has been reported to play a role in injury recoveryCitation23. rhEPO administration has been shown to make an important cytoprotective effect against cisplatin-induced oxidative damage and renal injury. They showed that rhEPO reduced MDA and protein carbonyl levels. rhEPO also prevented glutathione depletion and ameliorated the increased CAT activity induced by cisplatin treatmentCitation47.
Recently, it was shown that rhEPO decreased the ROS levels, the MDA levels and ameliorated glutathione modulation induced by cisplatin and mitomycin C in cultured Vero cellsCitation48.
Vitamin E is an essential nutrient that functions as a non-enzymatic antioxidant. It is an important biological free radical scavenger, which can exert its effect both on cells and membranes by protecting low-density lipoproteins and polyunsaturated fats in membranes from oxidationCitation49. Authors have shown the effect of vitamin E against gentamicinCitation14,Citation16,Citation50, vancomicinCitation51, cisplatinCitation52 and colistinCitation53. In the literature, there was no investigation about the effect of vitamin E against AK induced nephrotoxicity in rats.
In this study, renal tissue MDA and NO levels were decreased with the administration of vitamin E. Also, the levels of GPX in vitamin E group were increased significantly. An increase of SOD levels in AK-treated group was detected and also in AK + vitamin E treated group the SOD levels were decreased; but the results were not statistically significant.
Abdel-Naim et al.16 made a toxicity study with gentamicin and found that vitamin E ameliorated the rise in renal content of MDA and enhanced the renal SOD activity. Patel Manali et al.Citation50 also showed the gentamicin toxicity and reported that vitamin E and NAC significantly restored renal functions, reduced lipid peroxidation, enhanced reduced glutathione level. These results of gentamicin and vitamin E were similar with our study with AK and vitamin E.
In conclusion, one of the important mechanisms of AK nephrotoxicity is tubulointerstitial injury related to oxidative stress. This injury is supported with biochemical and renal histopathological findings. In the group treated with AK a significant increase of the MDA and NO levels as well as the decline of antioxidant enzyme GPX was shown to be statistically significant compared to the control group. Vitamin E and EPO, as antioxidants, can be useful renoprotective agents for ameliorating AK induced nephrotoxicity in neonates. To the best of our knowledge, this is the first study investigating the protective effect of EPO and vitamin E in case of AK induced nephrotoxicity on neonatal rats. In this regard, to better understand the preventive properties of EPO and vitamin E in neonates, more experimental or clinical studies are suggested.
Acknowledgments
This study has the approval of the animal ethics committee of the institution and conducted in accordance with standards such as the NIH Guide to the Care and Use of Laboratory Animals. All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Disclosure statement
The authors declare that there are no conflicts of interest.
Funding information
This work financially supported by the Süleyman Demirel University Research Fund (Project No: 1456-TU-06).
References
- Luft FC, Aronoff GR, Evan AP, Connors BA. The effect of aminoglycosides on glomerular endothelium: A comparative study. Res Commun Chem Pathol Pharmacol. 1981;34:89–95.
- Langhendries JP, Battisti O, Bertrand JM, et al. Once a day administration of amikacin in neonates: Assessment of nephrotoxicity and ototoxicity. Dev Pharmacol Ther. 1993;20:220–230.
- Klein J, Koren G, MacLeod SM. Comparison of methods for prediction of nephrotoxicity during development. Dev Pharmacol Ther. 1992;19:80–89.
- Provoost AP, Adejuyigbe O, Wolff ED. Nephrotoxicity of aminoglycosides in young and adult rats. Pediatr Res. 1985;19:1191–1196.
- Houghton DC, Plamp CE, Gilbert DN, et al. Amikacin nephrotoxicity in the rat. J Environ Pathol Toxicol. 1980;4:227–291.
- Rankin LI, Luft FC, Yum MN, Isaacs LL. Comparative nephrotoxicities of dibekacin, amikacin and gentamicin in a rat model. Antimicrob Agents Chemother. 1980;19:983–985.
- Rankin LI, Luft FC, Yum MN, Sloan RS, Dinwiddie CB, Isaacs LL. Comparative Nephrotoxicity of SCH 21420 and Amikacin in rats. Antimicrobial Agents and Chemotherapy 1979;16:491–194.
- Vasquez-Mendoza G, Vargas-Origel A, Ramos-Jimenez AC, Aguilar-Orozco G, Romeo-Gutierrez G. Efficacy and renal toxicity of one daily dose of amikacin versus conventional dosage regime. Am J Perinatol. 2007;24:141–146.
- Parlakpınar H, Özer MK, Sahna E, Vardı N, Cigremiş Y, Acet A. Amikacin-induced acute renal injury in rats: Protective role of melatonin. J. Pineal Res. 2003;35:85–90.
- Brion N, Barge J, Godefroy I, et al. Gentamicin, netilmicin, dibekacin, and amikacin nephrotoxicity and its relationship to tubular reabsorption in rabbits. Antimicrob Agents Chemother. 1984;25:168–172.
- Parlakpınar H, Koç M, Polat A, et al. Protective effect of aminoguanidine against nephrotoxicity induced by amikacin in rats. Urol Res. 2004;32:278–282.
- Parlakpınar H, Özer MK, Uçar M, et al. Protective effects of caffeic acid phenethyl ester (cape) on amikacin induced nephrotoxicity in rats. Cell Biochem Funct. 2006;24:363–367.
- Kaynar K, Gül S, Ersöz S, Özdemir F, Ulusoy H, Ulusoy S. Amikacin-induced nephropathy: Is there any protective way? Renal Fail. 2007;29:23–27.
- Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2005;90:571–576.
- Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc. 2007;39:864–865.
- Abdel-Naim AB, Abdel-Wahab MH, Attia FF. Protective effects of vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 1999;40:183–187.
- Nagai T, Akizawa T, Nakashima Y, et al. Effects of rHuEpo on cellular proliferation and endothelin-1 production in cultured endothelial cells. Nephrol Dial Transplant. 1995;10:1814–1819.
- Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47:740–745.
- Sharples EJ, Yaqoop MM. Erythropoietin in experimental acute renal failure. Nephron Exp Nephrol. 2006;104:83–88.
- Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and Mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55:808–820.
- Sharples EJ, Patel N, Brown P, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124.
- Bagnis C, Beaufils H, Jacquiaud C, et al. Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol Dial Transplant. 2001;16:932–938.
- Çetin H, Olgar Ş, Öktem F, et al. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin Exp Pharmacol Physiol. 2007;34:1181–1155.
- Armagan A, Kutluhan S, Yilmaz M, et al. Topiramate and vitamin E modulate antioxidant enzyme activities, nitric oxide and lipid peroxidation levels in pentylenetetrazol-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2008;103:166–170.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol. 1990;186:421–431.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Sjöberg A, Oscarsson J, Olofsson S-O, Edén S. Insulin-like growth factor-I and growth hormone have different effects on serum lipoproteins and secretion of lipoproteins from cultured rat hepatocytes. Endocrinology. 1994;135:1415–1421.
- Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. J Pharm. Pract. 2014;27:573–577.
- El Mouedden M, Laurent G, Mingeot-Leclercq MP, Taper HS, Cumps J, Tulkens PM. Apoptosis in renal proximal tubules of rats treated with low doses of aminoglycosides. Antimicrob Agents Chemother. 2000;44:665–675.
- Hottendorf GH, Barnett D, Gordon LL, Christensen EF, Madissoo H. Nonparallel nephrotoxicity dose–response curves of aminoglycosides. Antimicrob Agents Chemother. 1981;19:1024–1028.
- Notenboom S, Miller DS, Smits P, Russel FGM, Masereeuw R. Role of NO in endothelin-regulated drug transport in the renal proximal tubule. Am J Physiol Renal Physiol. 2002;282:458–464.
- Mohaupt MG, Elzie JL, Ahn KY, Clapp WL, Wilcox CS, Kone BC. Differential expression and induction of mRNAs encoding two inducible nitric oxide synthases in rat kidney. Kidney Int. 1994;46:653–665.
- Plato CF, Garvin JL. Nitric oxide, endothelin and nephron transport: Potential interactions. Clin Exp Pharmacol Physiol. 1999;26:262–268.
- Hortelano S, Castilla M, Torres AM, Tejedor A, Bosca L. Potentiation by nitric oxide of cyclosporin A and FK506-induced apoptosis in renal proximal tubule cells. J Am Soc Nephrol. 2000;11:2315–2323.
- Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis. 1997;30:311–333.
- Nakas-Ićindić E, Avdagić N, Mijanović M, et al. Nitric oxide in gentamicin-induced acute tubular necrosis in rats. Bosn J Basic Med Sci. 2005;5:70–74.
- Zhang Y, Wang L, Dey S, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15:10296–10333.
- Ruschitzka FT, Wenger RH, Stallmach T, et al. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci USA. 2000;97:11609–11613.
- Kanagy NL, Perrine MF, Cheung DK, Walker BR. Erythropoietin administration in vivo increases vascular nitric oxide synthase expression. J Cardiovasc Pharmacol. 2003;42:527–533.
- Quaschning T, Ruschitzka F, Stallmach T, et al. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. Faseb J. 2003;17:259–261.
- Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3:011619.
- Kaynar K, Aliyazioglu R, Ersoz S, et al. Role of erythropoietin in prevention of amikacin-induced nephropathy. J Nephrol. 2012;25:744–749.
- Yalcin S, Müftüoğlu S, Cetin E, et al. Protection against cisplatin-induced nephrotoxicity by recombinant human erythropoietin. Med Oncol. 2003;20:169–173.
- Rjiba-Touati K, Boussema IA, Belarbia A, Achour A, Bacha H. Protective effect of recombinant human erythropoietin against cisplatin-induced oxidative stress and nephrotoxicity in rat kidney. Int J Toxicol. 2011;30:510–517.
- Rjiba-Touati K, Ayed-Boussema I, Soualeh N, Achour A, Bacha H, Abid S. Antioxidant and antigenotoxic role of recombinant human erythropoeitin against alkylating agents: Cisplatin and mitomycin C in cultured Vero cells. Exp Biol Med (Maywood). 2013;238:943–950.
- Halliwell B, Gutteridge GM. Antioxidants Defenses, Free Radical in Biology and Medicines. 3rd ed. New York: Oxford University; 1999:105–245.
- Patel Manali B, Deshpande S, Shah G. Evaluation of efficacy of vitamin E and N-acetyl cysteine in gentamicin-induced nephrotoxicity in rats. Ren Fail. 2011;33:341–347.
- Naghibi B, Ghafghazi T, Hajhashemi V, Talebi A, Taheri D. The effect of vitamin E in prevention of vancomycin-induced nephrotoxicity in rats. Res Pharm Sci. 2006;2:104–111.
- Ajith TA, Usha S, Nivitha V. Ascorbic acid and alpha-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: A comparative study. Clin Chim Acta. 2007;375:82–86.
- Ghlissi Z, Hakima A, Silab A, et al. Evaluation of efficacy of natural astaxanthin and vitamin E in prevention of colistin-induced nephrotoxicity in the rat model. Environ Toxicol Pharmacol. 2014;37:960–966.