Abstract
Diabetes is the leading cause of end-stage renal disease because diabetic nephropathy (DN) develops in 30–40% of the patients. This study investigated the protective effect of the aqueous extract from leaves of Cyclocarya paliurus (Batal.) Iljinsk (ACP) on DN by inhibiting oxidative stress and aldose reductase (AR) activity. ACP was obtained by hot water extraction. The in vitro antioxidant capability and AR inhibition of ACP were investigated by employing various established systems. DN rats were used to assess the reno-protective effect of ACP. Results showed that the polysaccharide and total polyphenol contents of ACP were (479.3 ± 19.8) mg/g and (38.3 ± 2.3) mg/g, respectively. ACP exhibited strong antioxidant ability and AR inhibition in vitro and in vivo; furthermore, the inhibition mechanism of ACP in AR takes the form of uncompetitive inhibition. In addition, the animals treated with ACP showed significant amelioration of blood glucose, serum biomarkers related to renal function, urinary protein excretion, and histopathological changes in the kidney. The results suggest that ACP has a potential role in ameliorating renal damage involved in DN.
Introduction
Diabetic nephropathy (DN) is a clinical syndrome in patients with diabetes mellitus (DM) that is characterized by persistent hyperglycemia, worsening proteinuria, hypertension, and progressive renal failure. The blood glucose levels of a diabetic can be controlled within the normal range using insulin and other oral hypoglycemic agents, but DN develops in 30–40% of the patients.Citation1 With the rapidly rising number of DM worldwide, DN is becoming one of the leading causes of end-stage renal disease (ESRD). The pathogenesis of DN is complex, and an increasing body of evidence shows that oxidative stress (OS) has a pivotal role in the development and progression of DN.Citation2 The increased superoxide productions caused by the metabolic abnormalities of DM are the central and major mediators of microvascular injury in DN, and they trigger the activation of numerous pathways involved in the pathogenesis of DN, such as the polyol pathway (PP), one of the vital pathways of glucose metabolism in organisms.Citation3,Citation4 Aldose reductase (AR) is the key rate-limiting enzyme of PP, that is, AR inhibitors can inhibit AR activity and interfere with PP metabolism and therefore interfere with the occurrence and development of DN. Studies on OS and AR inhibition will contribute to the prevention and therapy of DN.Citation5–7
Many chemicals work with some or other pathway in the pathogenesis of DN and are characterized by weak specificity and considerable side effect. With respect to OS, traditional herbal medicines show particular superiority and features, such as Toona sinensis extract, Ginkgo biloba extract, Puerarin et al., which have been proven to intervene in the OS injury in DN rats.Citation8–10 Cyclocarya paliurus (Batal.) Iljinsk (abbreviated as CP), commonly known as “sweet tea tree,” is a kind of medicinal herb that is widely used in China to treat DM. In addition, CP antihyperglycemic herbal tea has been approved by the United States Food and Drug Administration (FDA) and is the first health tea from China certificated by FDA.Citation11 Many studies have demonstrated that CP possesses a variety of bioactivities, including antihypertensive, hypoglycemic, hypolipidemic, and antioxidant activity.Citation12,Citation13 However, no report is available on the effects of CP as an AR inhibitor, and no extensive research has been conducted on the effect of CP on DN. In this study, we investigate the antioxidant activity and AR inhibition of the aqueous extract of leaves of CP (ACP) in vitro and in vivo. Furthermore, the mechanism of the AR inhibition is analyzed, and the reno-protective effect of ACP is manifested in DN model rats. We also measure the polysaccharide and total polyphenol contents of ACP because they are the major bioactive substances.Citation14
Materials and methods
Chemicals
Epalrestat (standard, content > 99.4%, National Institutes for Food and Drug Control, China), NADPH and DL-glyceraldehyde (Shanghai Yuanye Bio-technology Co., Ltd. China), STZ (Sigma Co., St. Louis, MO), rat insulin ELISA kit (EMD Millipore Co., Billerica, MA), rat IL-6 and endothelin ELISA kit (NEO Bioscience Technology, Beijing, China), and antioxidant capacity assay kits used in the study were all purchased from Jiancheng Bioengineering Institute, Nanjing, China. All the other chemicals and reagents used were of analytical grade.
Plant material and preparation of ACP
Cyclocarya paliurus (Batal.) (CP) leaves were collected in Zhangjiajie, Hunan province, China and authenticated by Dr. Chongmei Xu of the Department of Pharmacognosy of Weifang Medical University, China. A voucher specimen (No. 20131102) was deposited in the herbarium of the university. The air-dried leaves were extracted with 20 parts of pure water (2 × 2 h), and the ACP was used in the research. The extracts were immediately dissolved in ultra pure water before the experiments.
Animals
Male Wistar albino rats weighing between 180–220 g and New Zealand white rabbits were all provided by the Weifang Medical Experimental Animal Center and were bred in a standard animal facility. The animals were kept under controlled conditions and fed with standard pellet diet and water ad libitum. All the experiments on the animals were conducted in accordance with and were approved by the Institution Animal Ethics Committees of Weifang Medical University.
Determination of polysaccharide and total polyphenol contents
The polysaccharide contents were determined using the phenol-sulfuric acid colorimetry method with d-glucose as standard, and the total polyphenol contents were detected using the Folin-phenol colorimetry method with gallic acid as standard.
In vitro antioxidant activity of ACP
Determination of total antioxidant capability
The total antioxidant capability (T-AOC) assay kit and microplates were used for quantitative assay. Ascorbic acid was used for positive control. T-AOC of ACP was calculated by the following equation derived from the kit’s description:
Determination of DPPH radical scavenging
The DPPH radical scavenging assay was conducted by means of an improved method.Citation15 Briefly, ACP with different concentrations were mixed with the same volume of DPPH solution (0.2 mM, in absolute alcohol), and the mixtures were placed away from light for 15 min. The absorbance was detected immediately at 515 nm.
Determination of capability of scavenging superoxide anion and hydroxyl radical
Anti-superoxide anion and hydroxyl radical kits were used with ascorbic acid as standard to determine the ACP’s capability of scavenging radicals.
Inhibitory effect of ACP on AR
Preparation of AR
The rabbits were anesthetized, and the crystalline lenses were separated. Two lenses were cut in a centrifuge tube, and 1 ml of pre-cooled distilled water was added. They were sonicated in an ice bath (200 w, 9 s × 6) to homogenize them and then centrifuged at 4 °C (l7,465 g × 30 min). The colorless and transparent supernatant was taken and placed at 4 °C for usage within 24 h.
Determination of the inhibitory effect of ACP and PCP on AR
A 20 mg/mL solution of ACP was precipitated three times as its volume of 95% ethanol. After filtering and freeze drying, the crude polysaccharide of CP (abbreviated as PCP) was obtained.
The reaction was conducted in a 96-well ELISA plate, and three repeated wells were set for each group with epalrestat (EP) as positive control.Citation16,Citation17 ACP and PCP were dissolved with ultra pure water at different concentrations. The blank reaction consisted of 10 μl of enzyme preparation. The standard reaction and the control consisted of 80 μl of 0.16 mM NADPH and 10 mM dl-glyceraldehyde (substrate, dissolved by NADPH), respectively. The test sample consisted of all the components of the control along with 10 μl of different concentrations of sample solutions, individually. The blank sample was used to correct the absorbance of the sample solutions, and the final volume was made up of 100 μl with sodium phosphate buffer (pH 6.4). The BioTek microplate reader (Gene Co., Ltd., Chai Wan, Hong Kong) monitored the absorbance of NADPH at 340 nm for 20 successive min. The enzyme activity at 60% to 70% time point was taken to calculate the AR inhibition rate of the samples. The formulas used are as follows.
Determination of inhibition type on AR
Based on the method above, the concentrations of the substrate dl-glyceraldehyde were 2.0, 2.5, 3.3, 5.0, and 10 mM. A decrease in NADPH absorbance was recorded at every minute. A double-reciprocal plot was used to determine the inhibition type, and the Michaelis–Menten equation was used to calculate the kinetic constants of the inhibitory activity on AR.Citation18
In vivo experiments
Ten Wistar rats were selected as the normal control group (group 1). The remaining rats were fed with a high-fat diet (HFD) for 6 weeks and were injected intraperitoneally with a dose of 35 mg/kg streptozotocin (STZ) in citrate buffer (pH = 4.5). Blood was drawn from the tail vein after 72 h to measure the blood glucose levels. The animals with blood glucose fewer than 10 mM were subjected to fasting and injected again. After a period of five weeks, the rats with blood glucose >16.7 mM, urine output >1.5 times as much as the original amount, and urinary albumin excretion rate >20 μg/min were used for study. The animals were then divided into the following six groups according to their blood glucose gradient: Group 2 (20 rats): DN model; Groups 3 and 4 (12 rats each): DN rats treated with ACP (0.047 and 0.094 g/kg/day, respectively; the dose used was determined by the conversion ratio of rats to human and the yield of ACP); and Group 5 (12 rats): DN rats treated with ascorbic acid (0.075 g/kg/day). The rats in Groups 1 and 2 were treated with an equal volume of distilled water. Each group was administered by gavage once a day every evening for 8 weeks. The general status, body weight, food intake, blood glucose, urine output, and urine protein excretion were determined regularly, and a metabolic cage was used to collect urine within 24 h.
At the end of the treatment, all the rats were anesthetized, and the serum was separated for biochemical and anti-oxidative analysis. An automatic biochemistry analyzer was used as an auxiliary measuring instrument to detect blood glucose (BG), serum creatinine (SCr), blood urea nitrogen (BUN), and 24 h urine protein (Upro), urine glucose (UG), urine creatinine (UCr), cystatin C(CysC), α1 microglobulin(AMG) levels. Rat insulin, endothelin, and IL-6 ELISA kits were used to determine the serum insulin (SI), endothelin-1 (ET-1), and interleukin-6 (IL-6). Several OS indicators, such as catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), malondialdehyde (MDA), and T-AOC, were detected with commercial kits. The kidneys were collected and weighed. A piece of cortex renis was frozen immediately in liquid nitrogen and stored at −80 °C until analysis, and the rest of the kidneys were preserved in 40% paraformaldehyde. The renal index (RI) was calculated with the following equation: RI (%) = [kidney weight (g)/body weight (g)] × 100%.
Statistical analysis
The experimental results were subjected to variance analysis using SPSS version 16.0 (SPSS Inc., Chicago, IL) and expressed as mean ± SD.
Results
Determination of polysaccharide and total polyphenol contents
The yield of ACP was 4.98%. The polysaccharide and total polyphenol contents of ACP were (479.3 ± 19.8) mg/g and (38.3 ± 2.3) mg/g, respectively.
In vitro antioxidant activity of ACP
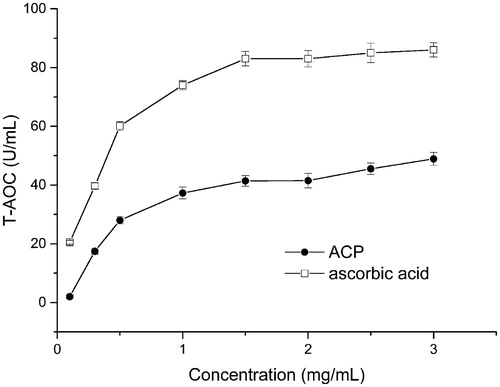
ACP showed a property to scavenge DPPH, superoxide anion, and hydroxyl radicals in varying degrees. The IC50 of ACP and ascorbic acid on three radicals are listed in , and the T-AOC of ACP at different concentrations is shown in . The results showed that ACP had good antioxidant activity and could well scavenge DPPH and hydroxyl radicals.
Table 1. IC50 of ACP and ascorbic acid on three radicals (n = 5).
Inhibitory effect of ACP on AR
Determination of rabbit lens AR activity
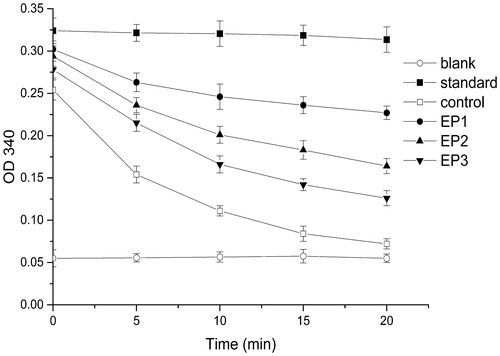
NADPH has a single absorption peak at 340 nm. The reaction of AR to the substrate consumes NADPH, thereby decreasing the absorption at 340 nm. shows that the absorbance was unchanged in the standard group because no substrate was added and that it declined in the control group. However, EP exhibited a significant inhibition on AR, which demonstrated that the reaction system was stable and reliable.
Inhibitory effect of ACP and PCP on AR
The results showed that ACP and PCP could inhibit AR in varying degrees and that the performance of ACP was remarkable with IC50 = 0.13 mg/mL, which was significantly better than that of PCP (IC50 = 1.13 mg/mL).
Inhibitory mechanism of ACP on AR
The results indicated that, as the concentration of the substrate increased, the reaction rate grew. The AR catalysis of the substrate decreased as the ACP concentration increased (r > 0.99), but the gradient remained unchanged, which suggested that the inhibitory effect of ACP on AR took the form of uncompetitive inhibition, that is, the inhibitors combined with the enzyme–substrate to inhibit the enzymatic reactions. The values of the Michaelis constant (Km) and maximum reaction rate (Vmax) are shown in . The Km value indicated the high affinity of the enzyme substrate, and the decline in Km when the inhibitor was added confirmed the uncompetitive inhibition.
Table 2. Kinetic properties of rabbit lens AR.
General features of the experimental rats
Compared with those in the normal control, DN rats had tarnished fur and anabrotic tails with the following symptoms: polyphagia, polydipsia, polyuria-thickened urine smell, and other signs of DN. Their body weight decreased after a period of increase caused by the abundant nutrition of HFD. Treatment of ACP improved these general features and depressed their mortality rate compared with the DN group.
Effects of ACP on renal function
The results are summarized in . The increase in BG, decrease in SI, and other variations in blood and urine analysis indicated the failure of the renal function. The treated groups notably improved compared with the DN group in terms of these indicators. The effects of ACP were better than the positive control.
Table 3. Results of blood and urine analysis in DN rats.
Effects of ACP on oxidative stress
The oxidative stress systems were determined as shown in . The variations in these OS indicators were found to be significant versus the blank control, which indicated OS in DN rats. In contrast, the OS injuries were found to be significantly improved in the ACP- and ascorbic acid-treated groups versus in the DN group. The results suggested that the ACP might have effective anti-oxidative properties in vitro and in vivo.
Table 4. Effects of ACP on antioxidant activity in DN rats.
Effects of ACP on renal AR activity
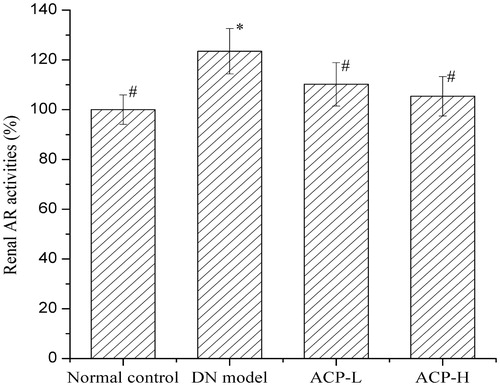
Kidney homogenate was centrifuged, and the supernatant was used for analysis. A regular reaction time was set, and the enzyme activity of the blank group was used reference of 100%. shows that the renal AR activity in DN rats was obviously enhanced compared with that in normal rats. This enhancement was markedly suppressed by ACP treatment.
Histopathological findings
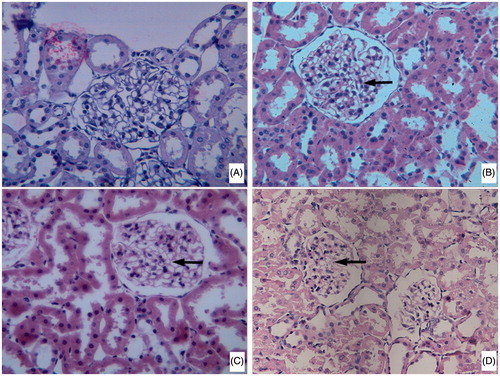
Light microscopy of HE-stained sections showed the damage of the renal tissue in the DN group, which thickened much in the glomerular basement membrane, increased in the mesangial matrix material, and expanded in the mesangial. The above histopathological changes in the ACP treatment groups were attenuated in a dose-dependent manner ().
Discussion
The STZ-induced renal pathophysiological changes and deteriorated functions that occur in animals under hyperglycemic conditions are very similar to those in human DM.Citation19 In this study, a second injection of STZ is given to the undesirable rats, and this approach has a success rate over 90% and is conducive to the extremely efficient use of experiment animals. A high level of blood glucose and low level of SI are observed in the experimental rats. The RI, BUN, SCr, and Upro all significantly increase in the DN group, together with the pathologic changes that indicate the formation of DN. Nevertheless, BUN and SCr concentrations are affected by many factors, so that there is strong evidence supporting the need for the early detection of DN when timely intervention can improve the long-term outcome.Citation20,Citation21 Cys C is emerging as a better estimator of glomerular filtration rate than SCr. The increased levels of Cys C in diabetes has been reported as a possible early indicator of incipient DN, and even minimal glomerular damage results in a significant increase in serum Cys C.Citation22 AMG is a glycoprotein in blood and has been studied as a sensitive marker for renal tubular dysfunction in various disorders. Furthermore, the link of the pro-inflammatory cytokines IL-6 and ET-1 to various diabetic complications has been demonstrated. A number of clinical studies have indicated a significant correlation between the serum concentration of ET-1 and the severity of albuminuria.Citation23 Animal studies have shown that ET-1 is not only more markedly expressed in the kidney of DM rats but can also mediate the effect of hyperglycemia on mesangial cell hypertrophy and synthesis of extracellular matrix proteins.Citation24 IL-6 as a candidate cytokine has a major role in the beginning and progression of DN.Citation25 Increased levels of IL-6 and other proteins are detected as early as stages 1–2 of the chronic kidney disease prior to significant kidney impairment.Citation26 Clinical studies have also reported that serum levels of IL-6 are associated with hypertension and atherosclerosis in patients with early stages of type 2 DN.Citation27 In this study, we find that several cytokines and biomarkers are significantly altered in experiment animals. Treatment with ACP decreases the blood glucose level and reduces the RI, BUN, SCr and Upro, as well as the Cys C, AMG levels and pro-inflammatory cytokines IL-6 and ET-1. These findings suggest that ACP can effectively ameliorate renal injury in DN rats and that the anti-inflammatory effect of ACP can be considered to be one of the renal protective mechanisms.
The T-AOC of a compound may serve as a significant indicator of its potential antioxidant activity, DPPH, and superoxide anion; hydroxyl radicals are all well known to be harmful to cellular components. The results show the antioxidant capability of ACP, although it is inferior to ascorbic acid. Under normal physiological conditions, a balance exists in the generation of oxygen free radicals and antioxidant defense systems used by organisms to deactivate and protect themselves against free radical toxicity.Citation28 The antioxidant defense system includes ROS degrading molecules, such as uric acid, ascorbic acid, glutathione, and antioxidant enzymes, such as CAT, GSH-Px, and SOD. In addition, the increase in MDA content reflects the degree of lipid peroxides. Some authors have reported that the expression of redox enzyme decreases and the expression of oxidase increases in diabetic models.Citation29,Citation30 The expression of oxidative stress-related cytokines is suppressed with the intervention of polyphenols.Citation31,Citation32 In the present research, OS is observed in DN rats at the end of the experiment, and ACP with excellent antioxidant capacity shows promising therapeutic potential in preventing the development of DN. We also found that the changes in the activities of the OS-related enzyme are consistent with many reports. We also detected polyphenols in ACP, and we believe that the antioxidant mechanism of ACP may exert effects on the related redox enzyme expression. Furthermore, this research provides a material base for the efficient separation and purification of natural antioxidant components.
In the diabetic, who is affected by high glucose level in vivo, carbohydrate oxidation increases, and the PP and other pathways are activated. This in turn leads to the unbalanced oxidation and anti-oxidization of organisms and excessive active oxygen radicals, which in turn damage tissues and cells and finally result in DN and other DM complications. Influenced by sustained hyperglycemia, AR activity increases significantly and activates PP, which enables many metabolites, including sorbitol, to accumulate in cells, thereby affecting cellular structures and functions and finally triggering a series of pathological changes. In our present study, renal AR activity can be effectively reduced by ACP in vitro and in vivo, and this suggests that ACP can suppress PP and alleviate renal failure in DN. Furthermore, this research provides new insights for the investigation of the antioxidation and AR inhibition of natural herb medicine in preventing and treating of DN.
In fact, many synthetic ARIs have been developed as drug candidates, but most of them have been virtually unsuccessful in clinical trials because of adverse pharmacokinetic properties, inadequate efficacy, and toxic side effects. By contrast, ACP, as a natural product has the advantages of high efficiency and low toxicity. The results of ACP and PCP on AR indicate that PCP is not the major effective substance in ACP on AR. The presumption that some other constituents take effect in ACP should inspire us to conduct further research.
Conclusion
Iljinsk (ACP) shows significant antioxidant and AR inhibitory activity in vitro and in vivo. The protective effects of ACP on reducing STZ induce hyperglycemia, and the associated renal injury may be related to the effect of ACP on reducing oxidative stress as well as on suppressing the activation of the polyol pathway through AR inhibition. However, further investigations need to be conducted to justify the active constituents of ACP for DN treatment and to study its mechanism in depth.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding information
This work was supported by the National Natural Science Foundation of China (81274049) and the Traditional Chinese Medicine Science and Technology development projects of Shandong province (2015–231 and 2015–235).
References
- Valk EJJ, Bruijn JA, Bajema IM. Diabetic nephropathy in humans: Pathologic diversity. Curr Opin Nephrol Hypertens. 2011;20:285–289.
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070.
- Feng B, Ruiz M, Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can J Physiol Pharmacol. 2013;91:213–220.
- Jang S, Kim M, Choi M, Kwon E, Lee M. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metab Clin Exp. 2010;59:512–519.
- Schemmel K, Padiyara R, Souza J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24:354–360.
- Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos V. Aldose reductase enzyme and its implication to major health problems of the 21(st) century. Curr Med Chem. 2009;16:734–752.
- Srivastava S, Ramana K, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392.
- Yang H, Chen S, Lin K, Wang MT, Chen YC, Huang HC, Cho HJ, et al. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J Ethnopharmacol. 2011;7:669–680.
- Ren M, Yang S, Li J, Hu Y, Ren Z, Ren S. Ginkgo biloba L. extract enhances the effectiveness of syngeneic bone marrow mesenchymal stem cells in lowering blood glucose levels and reversing oxidative stress. Endocrine. 2013;43:360–369.
- Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo K, Kim JS. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol Med. 2012;53:357–365.
- Wang QQ, Jiang CH, Fang SZ, Wang J, Ji Y, Shang X, Ni Y, et al. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J Ethnopharmacol. 2013;150:1119–1127.
- Xie JH, Liu X, Shen MY, Nie SP, Zhang H, Li C, Gong DM, Xie MY. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013;136:1453–1460.
- Kurihara H, Asami S, Shibata H, Fukami H, Tanaka T. Hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol Pharm Bull. 2003;26:383–385.
- Xie JH, Liu X, Shen MY, Nie SP, Zhang H, Li C, Gong DM, Xie MY. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr Polym. 2012;89:177–184.
- Fu R, Zhang YT, Guo YR, Huang QL, Peng T, Xu Y, Tang L, Chen F. Antioxidant and anti-inflammatory activities of the phenolic extracts of Sapium sebiferum (L.) Roxb. leaves. J Ethnopharmacol. 2013;147:517–524.
- Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, Hong EK Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J Ethnopharmacol. 2005;101:49–54.
- Li C, Ng K, Shao Y , Liu XB, Ling CC, Ma YY, Geng W, et al. The inhibition of aldose reductase attenuates hepatic ischemia-reperfusion injury through reducing inflammatory response. Ann Surg. 2014;260:317–328.
- Kumar R, Patel D, Laloo D, Sairam K, Hemalatha S. Inhibitory effect of two Indian medicinal plants on aldose reductase of rat lens in vitro. Asian Pac J Trop Med. 2011;4:694–697.
- Manna P, Sinha M, Sil P. Prophylactic role of arjunolic acid in response to streptozotocin mediated diabetic renal injury: Activation of polyol pathway and oxidative stress responsive signaling cascades. Chem Biol Interact. 2009;181:297–308.
- Jeon YL, Kim MH, Lee WI, Kang SY. Cystatin C as an early marker of diabetic nephropathy in patients with type 2 diabetes. Clin Lab. 2013;59:1221–1229.
- Matys U, Bachorzewska-Gajewska H, Malyszko J, Dobrzycki S. Assessment of kidney function in diabetic patients. Is there a role for new biomarkers NGAL, cystatin C and KIM-1? Adv Med Sci. 2013;58:353–361.
- Sun Q, Shen ZY, Meng QT, Liu HZ, Duan WN, Xia ZY. The role of DJ-1/Nrf2 pathway in the pathogenesis of diabetic nephropathy in rats. Ren Fail. 38(2):294–304.
- Žeravica R, Čabarkapa V, Ilinčić B, Sakač V, Mijović R, Nikolić S, Stošić Z. Plasma endothelin-1 level, measured glomerular filtration rate and effective renal plasma flow in diabetic nephropathy. Ren Fail. 2015;37:681–686.
- Zanatta CM, Veronese FV, Loreto MS, Sortica DA, Carpio VN, Eldeweiss MI, da Silva VD, et al. Endothelin-1 and endothelin a receptor immunoreactivity is increased in patients with diabetic nephropathy. Ren Fail. 2012;34:308–315.
- Svensson MK, Eriksson JW. Change in the amount of body fat and IL-6 levels is related to altered insulin sensitivity in type 1 diabetes patients with or without diabetic nephropathy. Horm Metab Res. 2011;43:209–215.
- Perlman AS, Chevalier JM, Wilkinson P, Liu H, Parker T, Levine DM, Sloan BJ, et al. Serum inflammatory and immune mediators are elevated in early stage diabetic nephropathy. Ann Clin Lab Sci. 2015;45:256–263.
- Dimas G, Iliadis F, Tegos T, Spiroglou S, Kanellos I, Karamouzis I, Savopoulos C, et al. Serum levels of timp-1 and IL-6 are associated with hypertension and atherosclerosis in patients with early stages of chronic kidney disease and type 2 diabetic nephropathy. J Hypertens. 2015;33:e55.
- Arora MK, Singh UK. Oxidative stress: Meeting multiple targets in pathogenesis of diabetic nephropathy. Curr Drug Targets. 2014;15:531–538.
- Sadi G, Güray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: Effect of antioxidants. Mol Cell Biochem. 2009;327:127–134.
- Hou S, Zheng F, Li Y, Gao L, Zhang J. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int J Mol Sci. 2014;15:15026–15043.
- Tang DQ, Wei YQ, Gao YY, Yin XX, Yang DZ, Mou J, Jiang XL. Protective effects of rutin on rat glomerular mesangial cells cultured in high glucose conditions. Phytother Res. 2011;25:1640–1647.
- Hong SH, Lee HJ, Sohn EJ, Ko HS, Shim BS, Ahn KS, Kim SH. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol Rep. 2013;65:970–979.