Abstract
Curcumin and dexmedetomidine have been shown to have protective effects in ischemia–reperfusion injury on various organs. However, their protective effects on kidney tissue against ischemia–reperfusion injury remain unclear. We aimed to determine whether curcumin or dexmedetomidine prevents renal tissue from injury that was induced by hind limb ischemia–reperfusion in rats. Fifty rats were divided into five groups: sham, control, curcumin (CUR) group (200 mg/kg curcumin, n = 10), dexmedetomidine (DEX) group (25 μg/kg dexmedetomidine, n = 10), and curcumin–dexmedetomidine (CUR–DEX) group (200 mg/kg curcumin and 25 μg/kg dexmedetomidine). Curcumin and dexmedetomidine were administered intraperitoneally immediately after the end of 4 h ischemia, just 5 min before reperfusion. The extremity re-perfused for 2 h and then blood samples were taken and total antioxidant capacity (TAC), total oxidative status (TOS) levels, and oxidative stress index (OSI) were measured, and renal tissue samples were histopathologically examined. The TAC activity levels in blood samples were significantly lower in the control than the other groups (p < 0.01 for all comparisons). The TOS activity levels in blood samples were significantly higher in Control group and than the other groups (p < 0.01 for all comparison). The OSI were found to be significantly increased in the control group compared to others groups (p < 0.001 for all comparisons). Histopathological examination revealed less severe lesions in the sham, CUR, DEX, and CUR–DEX groups, compared with the control group (p < 0.01). Rat hind limb ischemia–reperfusion causes histopathological changes in the kidneys. Curcumin and dexmedetomidine administered intraperitoneally was effective in reducing oxidative stress and renal histopathologic injury in an acute hind limb I/R rat model.
Introduction
Ischemia in skeletal muscle unavoidable in the upper or lower extremities, particularly due to tourniquet application during a variety of surgeries, including orthopedic, musculoskeletal reconstructive procedures, and vascular surgeries.Citation1 Ischemia–reperfusion injury may occur after the re-introduction of oxygenated blood to ischemic tissues, due to released free oxygen radicals and activated neutrophils.Citation2 After acute inflammatory response, secondary organ dysfunction and finally organ failure might occur, due to free oxygen radicals and leukocyte aggregation. In addition, several animal studies showed that hind limb I/R injury affected several organs, including the kidneys, liver and lungs.Citation3–Citation5
The highly selective and potent α2-adrenergic agonist dexmedetomidine (DXM) is an effective sedative, anxiolytic, and analgesic agentCitation6 with protective effects against I/R injury in several organs, including the heart,Citation7 brain,Citation8 and kidneys,Citation9 probably due to the antioxidant and anti-inflammatory properties of the compound.Citation10–12
Curcumin (CUR) has a long history of therapeutic use in Indian and Chinese medicine and has several pharmacological properties, including antioxidant, anti-inflammatory, antiviral, antimicrobial, antifungal, as well as anticancer activities.Citation13 CUR has attenuated several types of organ injury (lung, renal, hepatic, heart, and ovary) in different I/R models.Citation14,Citation15
This study was designed to investigate the potential effects of CUR, DXM, or combined pre-treatment on a renal I/R injury in a hind limb rat model.
Materials and methods
This study was performed using male WistarAlbino rats, aged 4–8 weeks and weighing between 250 and 300 g. The animals were housed in temperature (21 ± 2 °C) and humidity (60 ± 65%) controlled rooms in which a 12/12 h light/dark cycle was maintained, with free access to food and water. The experiments were carried out at the Harran University Experimental Research Center.
Ischemia reperfusion injury model
The rats were randomly assigned to five experimental groups: sham, control (I/R; non-treated), CUR (CUR-treated I/R), DEX (DEX-treated I/R), and CUR–DEX (Cur + Dex treated I/R). The rats were anesthetized under aseptic conditions by an intramuscular injection of ketamine 87 mg/kg (Ketalar; Parke Davis, Eczacibasi, Istanbul, and Turkey) and Xylazine 13 mg/kg (Rompun; Bayer AG, Leverkusen, and Germany) and additional 25 mg/kg intraperitoneal ketamine was applied when needed.
The lower limb ischemia–reperfusion method was adapted from a previous report.Citation16 In the sham group (n:10), only laparotomy was performed. In the control group (n:10) hind limb ischemia was performed for 4 h followed by 2 h period of reperfusion, but no drug was given. Group CUR (n:10), DEX (n:10), and CUR/DEX (n:10) underwent hind limb ischemia and perfusion with the same protocol and also 200 mg/kg curcumin to Group CUR, 25 μg/kg dexmedetomidine to Group DEX, and 200 mg/kg curcumin plus 25 μg/kg dexmedetomidine to Group CUR/DEX were given intraperitoneally, 5 min before reperfusion. The time of ischemia and reperfusion and the dose of curcumin and dexmedetomidine were based on previous studies.Citation14,Citation17–19
At the end of the procedures, the rats were sacrificed following blood sampling, and renal tissues obtained from all of them. Mean operation time of groups was as follows; control group 21 ± 5.4 min, CUR group 22 ± 7.2 min, DEX group 21.5 ± 6.5 min, and CUR–DEX group 22 ± 2.25 min (p > 0.05). About 0.9% NaCl was IV administered through the tail vein at the dose of 5 mg/kg/h during ischemia–reperfusion period and during surgery for each group. Mean additional ketamine dosage of groups were as follows: control group 57 ± 2.2 mg, CUR group 56 ± 7.4 mg, DEX group 59 ± 4.1 mg and CUR–DEX group 58 ± 5.6 min. There were no statistically significant difference between groups (p > 0.05).
Curcumin (CUR) was dissolved in 1% dimethyl sulfoxide for intraperitoneal injection. CUR was purchased from Sigma Aldrich (Saint Louis, MO).
Histopathological evaluation
The organs were placed in formalin and embedded in wax according to standard protocols. Subsequently, they were sectioned at 5 mm slice thickness, and stained with hematoxylin and eosin. Magnification of 20× was used (Olympus BX51 TF, USA). Samples were then graded histologically according to the severity of injury, using a pre-determined scoring system. The pre-determined scoring system by Solez et al. included tubular necrosis, interstitial edema, loss of brush border, and cast formation in which the score was 0 for absent, 1 for mild to moderate, and 2 for marked renal involvement.Citation20
All the experiments in this study were performed in accordance with the guidelines for Animal Research from the National Institutes of Health and were approved by the Committee for Animal Research at Harran University, Sanliurfa, Turkey.
Biochemical analyses
Measurement of the total antioxidant capacity
The total antioxidant capacity (TAC) of supernatant fractions was determined using a novel automated measurement method developed by Erel.Citation21 Hydroxyl radicals, the most potent biological radicals, are produced using this method. In the assay, a ferrous ion solution present in Reagent 1, is mixed with hydrogen peroxide, which is present in Reagent 2. The radicals subsequently produced, such as brown-colored dianisidine radical cations produced by the hydroxyl radicals, are also against the potent-free radical reactions initiated by the hydroxyl radicals. The assay has excellent precision values lower than 3%. The results were expressed as nmol Trolox Equiv/mg protein.
Measurement of total oxidant status
The total oxidant status (TOS) of supernatant fractions was determined using a novel automated measurement method developed by Erel.Citation22 Oxidants present in the sample oxidize the ferrous ioneo-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules that are abundant in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of nmol H2O2 Equiv/mg protein.
Oxidative stress index
The percentage ratio of TOS level to TAC level was accepted as Oxidative Stress Index (OSI). OSI values were calculated according to the following formula.Citation22 OSI (arbitrary unit). TOS (nmol H2O2 Equiv/mg protein)/TAC (nmolTroloxEquiv/mg protein).
Statistical analysis
Statistical analyses were performed using SPSS version 11.5 (SPSS for Windows 11.5, Chicago, IL). Continuous data are expressed as mean ± SD, whereas categorical variables were presented as number (count) and percentage. Distribution of the continuous variables was assessed with the one-sample Kolmogorove–Smirnov test and indicated that all variables were abnormally distributed. For multiple comparisons, the Kruskal–Wallis test was used for comparisons between groups and the post-hoc Bonferroni test was used if any statistical significance was found. Histopathological scores were evaluated with Chi-Square test. A p values of <0.05 was considered statistically significant.
Results
The TAC activity levels in blood samples were significantly lower in the control than the other groups (p < 0.01 for all comparisons). The TAC activity levels in blood samples in CUR–DEX group were significantly higher than CUR and DEX groups (p < 0.01 for both comparisons). The TOS activity levels in blood samples were significantly higher in Control group and than the other groups (p < 0.01 for all comparison). The OSI were found to be significantly increased in the control group compared to others groups (p< 0.001 for all comparisons). Results of oxidative stress markers in each group are shown in .
Table 1. Oxidative and antioxidative parameters in sham, control, I/R + DEX, I/R + CUR, and I/R + DEX + CURI/R rats.
Histopathological damage scoring was statistically increased in the control group compared with other groups (p < 0.05 for all comparison). The histopathological image of the rat’s kidney in all groups is presented in .
Figure 1. (A) Histopathological evaluation of renal Sham Group: mild interstitial edema, and tubular cast formation and a loss of brush border showed in HEx20 focal areas. (B) Histopathological evaluation of renal control group: tubular necrosis is accompanied by significant loss of brush border, tubular cast formation, and mild interstitial edema were observed in HEX20 focal areas. C) Histopathological evaluation of renal DEX group: HEx20 tubular dilatation, interstitial edema, necrosis of tubular epithelial, and tubule epithelium lumen loss showed. (D and E) In both cases (CUR, CUR + DEX), a few selected of tubular necrosis and interstitial edema, mild tubular cast formation, and focal loss of brush border was observed focal areas in.
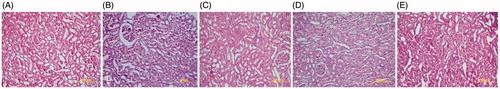
Histopathological examination of the tissues in the control group revealed lesions such as tubular damage characterized by the loss of brush border and cellular detachment from tubular basement membranes in the kidneys of the rats ().
Discussion
To the best of our knowledge, this is the first study evaluating the effect of curcumin, dexmedetomidine, and their combination on the kidney in hind limb I/R injury in a rat model. We have shown that (i) lower limb ischemia for 4 h followed by reperfusion for 2 h caused significant pathology in kidney tissue, (ii) TOS levels and OSI'es were statistically increased in the control group compared to others groups, (iii) The TAC activity levels in blood samples were significantly lower in the control than the other groups, (iv) CUR–DEX group TAC level was statistically increased compared to CUR and DEX group alone, (v) histopathological injury scores in kidney tissue were statistically decreased in the sham, CUR, DEX, and CUR–DEX groups compared to control group.
Limb ischemia–reperfusion is frequently encountered in operating theaters, especially with tourniquet usage to reduce bleeding, in orthopedic surgery. The skeletal muscle, which is the tissue most vulnerable to ischemia, can be subjected to prolonged ischemia and subsequent reperfusion, which might result in functional and metabolic changes.Citation23 Free radicals and ROS are key factors for I/R injury. In previous studies, the deleterious effects of these factors on various cell functions were shown, including mitochondrial oxidative phosphorylation, ATP depletion, activation of protein kinases, phosphatases, proteases, lipases, and nucleases, which might lead to loss of several cellular function and integrity.Citation24 This cellular dysfunction and disintegrity might play a role in the etiopathology of remote organ damage including liver, kidney and lung in lower limb ischemia–reperfusion injury.Citation3–5,Citation16
The kidneys are particularly involved in the remote alteration phenomenon following skeletal muscle ischemia–reperfusion injuries.Citation23 Although a diversity of mechanisms exist, oxidative stress, inflammatory response, and the apoptosis are believed to be the primary causes. In addition, the high metabolic demands of active tubular re-absorption make the kidney particularly vulnerable to ischemic injury.Citation25,Citation26
Curcumin, a phytochemical derived from the rhizome of the curcuma longa plant, has been shown to have anticancer, antimicrobial,Citation27 hepatoprotective,Citation28 and antihyperlipidemic properties.Citation29 Recent studies proved the ability of curcumin and related compounds to inhibit free radical generation in vitro and in vivo in different organs, such as the lungs, brain, and heartCitation30–32 and curcumin is also shown to inhibit neutrophil infiltration.Citation33 These effects are mediated through the regulation of various transcription factors, growth factors, inflammatory cytokines, protein kinases, and other enzymes.Citation34–36
The renal protective effects of CUR have also been evaluated in several settings, including nephrectomy, diabetic nephropathy, shock-wave lithotripsy patients, in drug induced renal injury, and finally in different ischemia and reperfusion models, and these renal protective effects seem to be related to the preservation of antioxidant enzymes and the prevention of oxidative stress, as well as the inhibition of mitochondrial dysfunction and the attenuation of inflammatory response.Citation37–39
Dexmedetomidine, a highly selective and potent α2 adrenoceptor agonist, has sedative, analgesic, sympatholytic, and hemodynamic stabilizing properties and has been widely investigated in a variety of ischemia models and shown to have protective effect on brain, lung, intestine, liver, cardiac, testicular, and kidney tissues in animal models.Citation40
Several human studies, focusing on antioxidant properties of Dex in I/R injury, have revealed conflicting results. Yagmurdur et al. showed that Dex significantly attenuated plasma hypoxanthine, malondialdehyde, creatine phosphokinase, and uric acid levels in upper-extremity surgery.Citation41 On the other hand, in another study in lower extremity surgeries Dex was found to be ineffective in reducing the effects of tourniquet-induced ischemia–reperfusion injury during general anesthesia.Citation42
Studies focusing on the renoprotective effects of Dex have gained popularity in recent years but their effects are shown to be eliminated by α2-adrenoreceptor antagonists. Several mechanisms of renoprotection, have suggested including cytoprotective effect in extracellular regulated protein kinases (ERK) signaling pathway and suppressing the activation of JAK2/STAT3 signaling pathway.Citation43
Two physically compatible and chemically stable substances might be used in combination to achieve additive or synergistic effects. On the other hand, the rationale for combining curcumin and dexmedetomidine would be questioned for reasons of the differing mechanisms of action of the two drugs and the difference in their durations of action. It is also argued that it is not logical to administer two drugs and to have to anticipate the unique adverse effects of each when monotherapy works just as well and presents only one set of potential adverse events. Before recommending a combination, combination also needs to be shown to offer a noticeable advantage over either agent alone. We examined the combination of these two drugs to achieve additive or synergistic effect in hind limb ischemia reperfusion. The current data in our study have shown that an intraperitoneal bolus injection of DEX, CUR and also a combination or the two, reduced oxidative stress and have antioxidant properties similar to previous studies. We think that these favorable effects on oxidative stress play a part in reducing the histopathologic score and might be one of the protective mechanisms.
Several limitations of this study should be considered: One of the potential limitations is the absence of a different way to administer the CUR and DEX, such as orally or intravenously, versus the intraperitoneal route. DEX and CUR only applied just before reperfusion, we did not have any groups evaluating the effects of these drugs before ischemia. The application time might also affect the results and application before ischemia might reveal different results. Another limitation is the absence of biochemical analysis of the different renal parameters.
In conclusion, hind-limb I/R injury in a rat model might be attenuated with intraperitoneally CUR, DEX, and CUR–DEX combination and they might have potential preventive role on organ systems from I/R injury particularly due to tourniquet application during a variety of surgeries, however further large scale studies are needed to verify/exclude the possible favorable effects of CUR and DEX.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akar H, Sarac A, Konuralp C, Yildiz L, Kolbakir F. Comparison of histopathologic effects of carnitine and ascorbic acid on reperfusion injury. Eur J Cardiothorac Surg. 2001;19:500–506.
- Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637–647.
- Sirmali R, Armağan A, Oånktem F, et al. Protective effects of erdosteine, vitamin E, and vitamin C on renal injury induced by the ischemia-reperfusion of the hind limbs in rats. Turk J Med Sci. 2015;45:33–37.
- Garbaisz D, Turoålczi Z, Füloånp A, et al. Postconditioning can reduce long-term lung injury after lower limb ischemia-reperfusion. Magy Seb. 2013;66:146–154.
- Chen LN, Yang XH, Nissen DH, et al. Dysregulated renin-angiotensin system contributes to acute lung injury caused by hind-limb ischemia-reperfusion in mice. Shock. 2013;40:420–429.
- Nacif-Coelho C, Correa-Saler C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat.. Anesthesiology. 1994;81:1527–1534.
- Yoshitomi O, Cho S, Hara T, et al. Direct protective effects of dexmedetomidine against myocardial ischemia-reperfusion injury in anesthetized pigs. Shock. 2012;38:92–97.
- Engelhard K, Werner CE, EberspaÅNcher E, et al. The effect of the α2-agonist dexmedetomidine and the N-methyl-d-aspartate antagonist S (+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–531.
- Cakir M, Polat A, Tekin S, et al. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren Fail. 2015;37:704–708.
- Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245.
- Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: A histopathologic study. Ren Fail. 2009;31:70–74.
- Kilic K, Hanci V, Selek S, et al. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178:223–232.
- Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864.
- Aydin MS, Caliskan A, Kocarslan A, et al. Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int J Surg. 2014;12:601–605.
- Sak ME, Soydinc HE, Sak S, et al. The protective effect of curcumin on ischemia reperfusion injury in rat ovary. Int J Surg. 2013;11:967–970.
- Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26:115–121.
- Sompamit K, Kukongviriyapan U, Nakmareong S, Pannangpetch P, Kukongviriyapan V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur J Pharmacol. 2009;616:192–199.
- Avci G, Kadioglu H, Sehirli AO, et al. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res. 2012;172:39–46.
- Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153.
- Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine. 1979;58:362–376.
- Erel O. A novel automated method to measure total antioxidant response against potent-free radical reactions. Clin Biochem. 2004;37:112–119.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111.
- Padowitz RA. Tourniquet-induced neuromuscular injury. A recent review of rabbit and clinical experimets. Acta Orthop Scand. 1991;245:1–33.
- Estebe JP, Davies JM, Richebe P. The pneumatic tourniquet: Mechanical, ischaemia-reperfusion and systemic effects. Eur J Anaesthesiol. 2011;28:404–411.
- Tugtepe H, Sener G, Biyikli NK, et al. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept. 2007;140:101–108.
- Teruya R, Fagundes DJ, Oshima CT, et al. The effects of pentoxifylline into the kidneys of rats in a model of unilateral hindlimb ischemia/reperfusion injury. Acta Cir Bras. 2008;23:29–35.
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153.
- Rajagopalan R, Sridharana S, Menon VP. Hepatoprotective role of bis-demethoxy curcumin analog on the expression of matrix metalloproteinase induced by alcohol and polyunsaturated fatty acid in rats. Toxicol Mech Methods. 2010;20:252–259.
- Reddy BV, Sivagama Sundari J, Balamurugan E, Menon VP. Antihyperlipidemic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione, a curcumin analog, on nicotine and streptozotocin treated rats. Mol Cell Biochem. 2010;335:249–254.
- Kunchandy E, Rao A. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–240.
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the antiinflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59.
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83.
- Araujo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–728.
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
- Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016.
- Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994;137:1–8.
- Trujillo J, Chirino YI, Molina-Jijoåln E, Andeålrica-Romero AC, Tapia E, Pedraza-Chaverriål J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013;1:448–456.
- Hammad FT, Al-Salam S, Lubbad L. Curcumin provides incomplete protection of the kidney in ischemia reperfusion injury. Physiol Res . 2012;61:503–511.
- Bayrak O, Uz E, Bayrak R, et al. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol. 2008;26:285–291.
- Lempiaåninen J, Finckenberg P, Mervaala EE, et al. Dexmedetomidine preconditioning ameliorates kidney ischemia-reperfusion injury. Pharmacol Res Perspect. 2014;2:e00045.
- Yagmurdur H, Ozcan N, Dokumaci F, Kilinc K, Yilmaz F, Basar H. Dexmedetomidine reduces the ischemia–reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33:941–947.
- Bostankolu E, Ayoglu H, Yurtlu S, et al. Dexmedetomidine did not reduce the effects of tourniquet-induced ischemia-reperfusion injury during general anesthesia. Kaohsiung J Med Sci. 2013;29:75–81.
- Si YN, Bao HG, Xu L, et al. Dexmedetomidine protects against ischemia/reperfusion injury in rat kidney. Eur Rev Med Pharmacol Sci. 2014;18:1843–1851.
