Abstract
Objective The occurrence of acute kidney injury (AKI) after cardiopulmonary bypass (CPB) can lead to morbidity and mortality. We hypothesized that cysteine-rich protein 61 (CYR61) and cystatin C (CysC) may be potential novel biomarkers of AKI after cardiopulmonary bypass. Methods Patients were classified into AKI and non-AKI group depending on serum creatinine. Levels of creatinine, CysC, and CYR61 were measured at five time-points before and within 48 h after the surgery. Results Fifty patients were included in the study. Serum creatinine pre-operative values were 74.0 ± 43.3 μmol/L in AKI group vs. 64.8 ± 17.9 μmol/L in non-AKI group. During 48 h, the values increased to 124.6 ± 67.2 μmol/L in AKI group (p < 0.001) but in non-AKI group they did not change significantly. Serum CysC values were significantly increased already 2 h after CBP in AKI group (949 ± 557 μg/L, p < 0.05) compared to non-AKI group (700 ± 170 μg/L). Pre-operative serum CYR61 tended to be lower in AKI group (12.4 μg/L) than in non-AKI group (20.3 μg/L), but 24 h after the surgery, the levels in AKI group tended to be higher than non-AKI group. Conclusion Serum CYR61 does not seem to be an early predictor of AKI in patients after cardiac surgery with CPB, but it might possibly identify patients at risk of developing more severe kidney injury. Serum CysC could be a promising biomarker of AKI, differentiating patients at risk of developing AKI after cardiac surgery as early as 2 h after surgery.
Introduction
Acute kidney injury (AKI) is a serious progressive problem that affects patients after cardiac surgery and increases the rate of morbidity and mortality considerably.Citation1–3 The incidence of AKI among hospital-acquired patients ranges from 5% in patients with normal kidney function up to 25% in ICU patients.Citation4,Citation5 The etiology of AKI has three major origins: pre-renal that might occur due to hypovolemia, renal hypoperfusion, coagulopathies, congestive heart failure, and cirrhosis; renal which resulted from glomerulonephritis, acute tubular necrosis, interstitial nephritis, and vasculopathies; and post-renal which can be developed from renal calculi, urinary tract obstruction, bladder malignancy, prostatic hyperplasia and blood clots.Citation6,Citation7 Therefore, early screening and diagnosis of AKI is an important step in preventing and optimizing therapy.Citation8 The classification of AKI is dependent on KDIGO criteria,Citation9 a new updated harmonized RIFLE (Risk, Injury, Failure, Loss, and ESRD) criteriaCitation10 and AKIN (Acute Kidney Injury Network) criteriaCitation11 and relies on serum creatinine within 48 h and urinary output if available.
The traditional AKI marker is creatinine which remains unchanged until 50% of kidney function falls down.Citation11 It is affected by non-specific factors like diet, age, dehydration, muscle mass, gender, and drugs; and these influences can result in poorer diagnosis and wasted time of treatment decisions.Citation12,Citation13 Cystatin C (CysC), a cystatin protease inhibitor, is less affected by non-specific factors. When GFR decreases by the action of catabolism in proximal renal tubules, CysC begins to increase.Citation14 CysC was recommended by several authors to be measured in addition to creatinine in GFR estimation.Citation15
Recent studies suggested that the concentration of CysC 24 h after surgeryCitation16 as well as pre-operative CysC valuesCitation17 were associated with AKI.
Cysteine-rich protein (CYR61) is an extracellular matrix molecule consisting of four distinct domains.Citation18 The secretion of CYR61 is relevant for different physiological functions including angiogenesis, fibrosis, migration, proliferation, differentiation, development, apoptosis, and senescence development.Citation19 CYR61 is massively abundant at sites of inflammation, wound healing, it is accompanied with chronic inflammation and tissue injury.Citation20 Furthermore, Quan et al. discovered that over-expression of CYR61 levels were possibly due to sensitivity to environmental stress, and/or hypoxic conditions.Citation21 Studies on mice after unilateral kidney ischemia reperfusion injury (IRI) suggested that blocking CYR61 could reduce the inflammatory consequences associated with ischemic AKI.Citation22 The data on serum CYR61 in AKI is scarce; therefore, the process of working on serum CYR61 may introduce more information about AKI which occurs after cardiac surgery with cardiopulmonary bypass (CPB).
This study aimed to evaluate whether the serum levels of CysC and CYR61 could be used as early biomarkers for the identification of patients who are prone to AKI before and after cardiac surgery with CPB.
Patients, materials. and methods
Patients
Our study included patients who were admitted to Department of Cardiovascular Surgery at the University Medical Center Ljubljana for elective cardiac surgery with CPB. The study was carried out according to the declaration of Helsinki. The National Medical Ethics Committee of the Republic of Slovenia approved the study protocol and an informed consent was obtained from all study participants prior to data collection. The inclusion criteria were ages from 20 to 80 years and normal kidney function as assessed by creatinine. The exclusion criteria were patients with diabetic nephropathy, lupus nephritis, overlapping syndromes, malignancies, pregnancy, and hemodialysis or kidney transplantation. The patients were classified into AKI group and non-AKI group according to serum creatinine measured before and within 48 h after the surgery using KDIGO classification.Citation9
Materials and methods
Blood samples were collected from all patients at five time-points which are: before surgery, at the end of CPB, 2, 24, and 48 h after the end of CPB. Thus, blood samples were collected in tubes without additives. After collection, samples were centrifuged to obtain serum. Serum aliquots were stored at –20 °C until analysis. Subsequently, serum creatinine was measured using automated assay based on modified kinetic Jaffe reaction (Siemens Healthcare Diagnostics Inc., Newark, DE) with limit of detection 9 μmol/L. For the measurement of serum CysC and CYR61 ELISA kits were used (Bio Vendor GmbH, Heidelberg, Germany and Elabscience Ltd, Wuhan, China, respectively). The limit of detection was 0.2 μg/L for CysC and 2 μg/L for CYR61. Moreover, Estimated GFR (eGFR) was calculated using Modification of Diet in Renal Disease (MDRD) formula.Citation23
The results were reported as mean ± standard deviation (SD) or median and interquartile ranges for cases without normal distribution. Statistical analysis was performed by a software package SPSS version 22 (SPSS Inc., Chicago, IL); p values <0.05 was considered to be significant. For creatinine and CysC, means were compared using Student t-test and ANOVA test with post-hoc Bonferroni analysis. Medians and interquartile ranges were obtained by non-parametric Mann–Whitney U test for CYR61. The performance of biomarkers was assessed by area under the curve (AUC) values.
Results
Fifty patients were classified into AKI group and non-AKI group according to KDIGO criteria dependent on serum creatinine values, data for all patients were summarized in . In AKI group, there were 18 patients with AKI stage 1 and 8 patients with AKI stage 2.
Table 1. A summary of clinical data of the patients.
Serum creatinine levels were significantly raised from the baseline in AKI group 2 h after CPB (93.3 ± 38.7 μmol/L) with p < 0.01 and reached the high peak plateau after 48 h of CPB in AKI group (124.6 ± 67.2 μmol/L) with p < 0.001. In non-AKI group, serum creatinine levels were significantly decreased from the baseline immediately after CPB (62.0 ± 16.4 μmol/L) with p < 0.01. Thereafter, there was a transient increase within 24 h (68.1 ±20.8 μmol/L) with p = 0.001, which dropped back to the pre-operative baseline through 48 h of CBP (64.6 ± 22.4 with p < 0.001 (). Serum creatinine values at different time points in AKI and non-AKI groups are shown in .
Figure 1. Serum creatinine values (mean and SD) at five time-points: before surgery, at the end of CPB and 2, 24, and 48 h after the end of CPB.
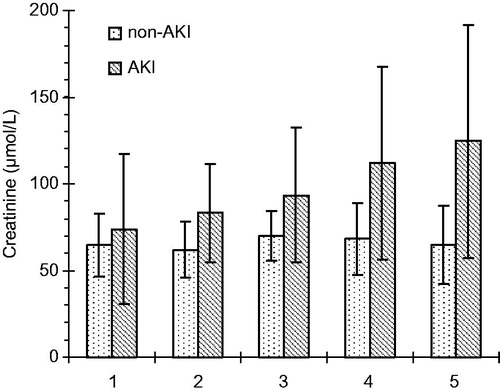
Table 2. Serum creatinine levels (μmol/L) in AKI and non-AKI groups at different points.
Serum CysC levels in AKI group gradually increased from the initial baseline to 2 h after CPB (949 ± 557 μg/L) with p < 0.05. They continued to increase to reach 1.5-fold the baseline within 48 h (1421 ± 739 μg/L) with p < 0.01, exhibiting similar dynamics to serum creatinine. However, a slight decline in serum CysC levels in non-AKI group was noticed 2 h after CPB (700 ± 170 μg/L) with p < 0.05 but then it persisted to increase up to (910 ± 422 μg/L) with p < 0.01 within 48 h as shown in . In addition, the serum CysC concentrations at different time points in AKI and non-AKI groups are presented in .
Figure 2. Serum CysC values (mean and SD) at five time-points: before surgery, at the end of CPB and 2, 24, and 48 h after the end of CPB.
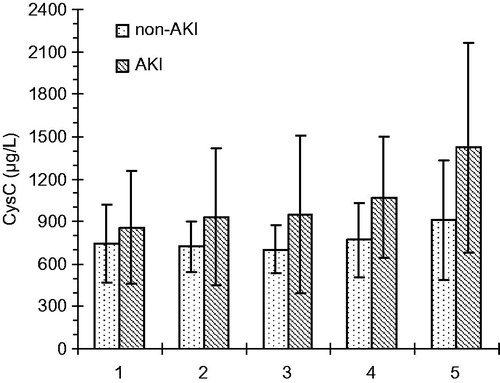
Table 3. Serum CysC levels (μg/L) in AKI and non-AKI groups at different points.
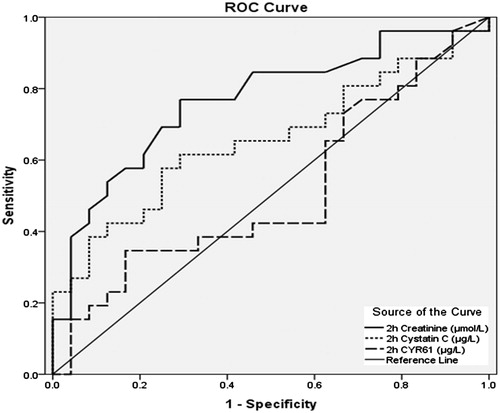
As CYR61 values were not normally distributed, medians and interquartiles were used instead of mean ± SD. The median baseline of serum CYR61 was higher in non-AKI group (20.3 μg/L) than in AKI group (12.4 μg/L) but the difference was not significant. Consequently, both groups experienced sudden drops in median values immediately after CBP and regained the pre-operative levels within 2 h (). After 48 h, the median values were higher than the baseline in AKI group but in the non-AKI group they reached not more than half the value of the baseline. A comparison of serum CYR61 median values between AKI and non-AKI groups did not show any significant difference as depicted in . However, 2 h after CPB, the comparison of groups with AKI stages 1 and 2 gave a lower p values, which was closer to significance (p = 0.066). Furthermore, a significant negative correlation was observed between serum CYR61 48 h after the end of CPB and age of the patients (p < 0.01, r = –0.465) as represented in . A receiver operating characteristic (ROC) curve analysis of the creatinine, CysC, and CYR61 values within 2 h after CPB gained the areas under the curve 0.767, 0.655, and 0.513, respectively, as shown in .
Figure 3. Serum CYR61 values (median and interquartile ranges) at five time-points: before surgery, at the end of CPB and 2, 24, and 48 h after the end of CPB.
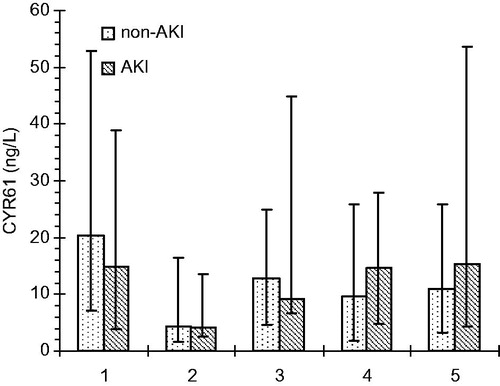
Figure 4. The correlation (r = −0.465; p < 0.01) between serum CYR61 levels (μg/L) 48 h after the end of CPB and age of the patients (years).
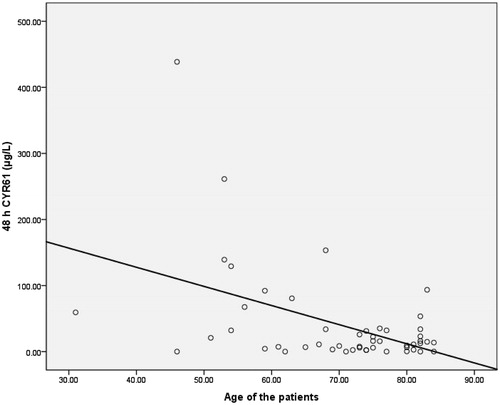
Table 4. Serum CYR61 levels (μg/L) in AKI and non-AKI groups at different points.
Discussion
Our findings revealed that serum creatinine levels increased more (p < 0.001) within 48 h post-operatively in AKI group than non-AKI group. However, the pre-operative serum creatinine baseline was higher in AKI group than non-AKI group. This difference might be explained by reduced pre-operative renal function with elevated state of inflammation due to endothelial dysfunction. These results are compatible with the study of Ishani et al., who mentioned that serum creatinine levels were increased in all patients after cardiac surgery, exceeding the pre-operative baseline.Citation24 Conversely, Sirota et al. showed that serum creatinine level was significantly decreased pre-operatively in AKI group in comparison to non-AKI group; and was overexpressed post-operatively in AKI group than non-AKI.Citation25 In contrast to adult population, a study which was carried out on pediatric cardiac patients by Peco-Antić et al. stated that no significant change was observed in serum creatinine baseline between AKI and non-AKI groups.Citation26
Rosner and Moran et al. explained the increase in CysC levels in AKI after CPB as a consequence of renal tubular damage which affected upstream glomerular filtration due to congestion.Citation27,Citation28 Accordingly, Zheng et al. noticed that post-operative serum concentrations of CysC in AKI group initially declined for 6 h, then subsequently rose again in 12 h, and got stabilized between 24 and 48 h after CPB.Citation29 In our study, serum CysC levels were decreased 2 h after CPB in non-AKI group only. In AKI group, we observed early significant increase of CysC levels.
Although CysC was recommended by some authors to be measured in addition to creatinine in GFR estimation,Citation15 it has not found its place in early detection of AKI after cardiac surgery. Creatinine, which had the highest AUC in our study, still seems to be the favorable single choice for early detection of AKI despite its limitations. A test panel including creatinine and new markers could add some value with improved specificity and fewer influences of all the factors that can affect creatinine results. Another challenge for the use of new markers remains the cost-effectiveness, but this important question exceeds the scope of our study.
Serum CYR61 baseline levels were found to be lower in AKI group than in non-AKI group. Although the difference was not significant, it is interesting due to the reversed ratio between AKI and non-AKI group on the first day after surgery, when serum CYR61 levels were higher than baseline in AKI group and lower than baseline in non-AKI group. The ratio was similar on the second post-operative day. This pattern is not yet well understood and the role of CYR61 in AKI needs to be further clarified.
We also noticed that serum CYR61 levels 2 h after CPB were different between the subgroups with AKI stage 2 and milder forms of AKI like AKI stage 1 (p = 0.066). The difference was not significant due to small number of patients in subgroups, but nevertheless the observation seems intriguing.
These findings suggest that serum CYR61 levels failed to discriminate AKI early after cardiac surgery with CPB. However, CYR61 might possibly identify patients with more severe kidney injury, which would be very beneficial for early treatment of AKI after cardiac surgery. With the results of our pilot study we cannot confirm this possible role of CYR61; further research involving larger groups of patients is needed.
Muramatsu et al. found that urinary CYR61 started to appear at 3–6 h, reached its maximum peak concentration at 6–9 h after kidney injury, and disappeared after volume depletion.Citation30 Volume depletion was accompanied with cardiac surgery by the effect of pump machine. Hence, this might also explain the drop of serum CYR61 level during CPB in our study. One of the strengths of our study is measuring CYR61 in serum samples, which usually give more reliable results than urine samples and also represent the true level of the analyte in the circulation at one given moment. To the best of our knowledge, no research on serum CYR61 in AKI after surgery using human samples has been published.
The broad inter-individual variations of CYR61 concentrations were reported by Hviid et al., who observed that CYR61 levels increased at sites of tissue injury like surgical wound closure which was characterized by inflammatory activation.Citation31 Great variability of CYR61 levels observed in our study could be at least partly influenced by similar factors and response to renal hypoperfusion without intensive ischemic acute kidney injury.Citation32 However, in some studies, CYR61 was activated by cytokine exposureCitation33 and promoter region of NF-κB binding-sitesCitation34 without de novo protein biosynthesis.Citation35
One of the limitations of our study is that some influences on the occurrence of AKI like serum lactate were not addressed. Although the causal relationship was not established, lactate was found to be independently associated with AKI.Citation36 These potential influences could be addressed in the future studies.
Conclusion
Although serum CYR61 does not seem to be an early predictor of AKI in patients after cardiac surgery with CPB, it might possibly identify patients at risk of developing more severe kidney injury, which would be very beneficial for early treatment. This possible role of CYR61 still needs further research involving larger groups of patients.
Serum CysC was found to differentiate patients at risk of developing AKI as early as two hours after cardiac surgery. The role of CysC as a marker of kidney function had been recognized by many studies, so this marker could be an added value in early evaluation of AKI after cardiac surgery using CPB, especially in combination with other markers.
Disclosure statement
All authors declare that they have no conflicts of interest.
Funding information
The study was financed by the UMC Ljubljana. There was no additional funding.
References
- Joseph LJ, Ryan AS, Sorkin J, et al. Body fat distribution and flow-mediated endothelium-dependent vasodilation in older men. Int J Obes Relat Metab Disord. 2002;26:663–669.
- Thakar CV, Liangos O, Yared JP, et al. ARF after open-heart surgery: Influence of gender and race. Am J Kidney Dis. 2003;41:742–751.
- Landoni G, Zangrillo A, Franco A, et al. Long-term outcome of patients who require renal replacement therapy after cardiac surgery. Eur J Anaesthesiol. 2006;23:17–22.
- de Mendonça A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921.
- Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–193.
- Vinay K, Nelson F, Nelso F, Stanley RL, Abul KA, Ramzi SC. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005:960–1012.
- Goljan EF. Rapid Review Pathology. 2nd ed. St. Louis, MO: Mosby, 2007:396–398.
- Endre ZH, Pickering JW. New markers of acute kidney injury: giant leaps and baby steps. Clin Biochem Rev 2011;32:121–124.
- Kidney Disease: Improving Global Outcomes (KDIGO). Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–141.
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Caregaro L, Menon F, Angeli P, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201–205.
- Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838.
- Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: A systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–365.
- Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: Incorporation into clinical practice. Am J Kidney Dis. 2013;62:595–603.
- Prowle JR, Calzavacca P, Licari E, et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail. 2015;37:408–416.
- Wang X, Che M, Xie B, Xue S, Yan Y. Preoperative serum cystatin C combined with dipstick proteinuria predicts acute kidney injury after cardiac surgery. Ren Fail. 2014;36:1497–1503.
- Wei L, Mckeon F, Russo JW, Lemire J, Castellot J. Domain-and species-specific monoclonal antibodies recognize the Von Willebrand Factor-C domain of CCN5. J Cell Commun Signal. 2009;3:65–77.
- Perbal B. CCN proteins: Multifunctional signalling regulators. Lancet. 2004;363:62–64.
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963.
- Quan T, He T, Shao Y, et al. Elevated Cysteine-Rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490.
- Lai CF, Lin SL, Chiang WC, et al. Blockade of cysteine-rich protein 61 attenuates renal inflammation and fibrosis after ischemic kidney injury. Am J Physiol Renal Physiol. 2014;11:670.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254.
- Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:1919.
- Sirota JC, Walcher A, Faubel S, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol. 2013;14:17.
- Peco-Antić A, Ivanišević I, Vulićević I, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem. 2013;46:1244–1251.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32.
- Moran SM, Myers BD. Pathophysiology of protracted acute renal failure in man. J Clin Invest. 1985;76:1440–1448.
- Zheng JY, Xiao YY, Yao Y, Han L. Is serum cystatin C an early predictor for acute kidney injury following cardiopulmonary bypass surgery in infants and young children? Kaohsiung J Med Sci. 2013;29:494–499.
- Muramatsu Y, Tsujie M, Kohda Y, et al. Early detection of cysteine rich protein 61 (CYR61, CCN1) in urine following renal ischemic reperfusion injury. Kidney Int. 2002;62:1601–1610.
- Hviid BVC, Pripp HA, Aasen OA, Danckert-Krohn C. Postoperative accumulation of CYR61/CCN1 in surgical wound fluid precedes cytokine activation and is disparate from systemic alterations. J Infect Dis Ther. 2014;2. DOI: 10.4172/2332-0877.1000181.
- Kanagasundaram NS. Pathophysiology of ischaemic acute kidney injury. Ann Clin Biochem. 2015;52:193–205.
- Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: A new class of inflammation modulators. Biochimie. 2011;93:377–388.
- Latinkic BV, O'Brien TP, Lau LF. Promoter function and structure of the growth factor-inducible immediate early gene CYR61. Nucleic Acids Res. 1991;19:3261–3267.
- O'Donnell A, Odrowaz Z, Sharrocks AD. Immediate-early gene activation by the MAPK pathways: What do and don't we know? Biochem Soc Trans. 2012;40:58–66.
- Zhang Z, Ni H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS One 2015;10:e0120466.