Abstract
Objectives IL-18 mediates various inflammatory and oxidative responses including renal injury, fibrosis, and graft rejection. It has been reported that the promoter -607 and -137 polymorphisms of IL-18 influence the level of IL-18. This prospective observational study investigated the association between oxidative stress with IL-18-607 and -137 polymorphisms in renal transplant recipients. Patients and methods This study included 75 renal transplant recipients (28 female, 47 male) from living-related donors. Blood samples were collected immediately before and after transplantation at day 7 and month 1. Serum IL-18, creatinine, cystatin C, CRP, and oxidative stress markers (TOS, TAC) were measured. The Oxidative Stress Index (OSI) was calculated. Polymorphisms of the promoter region of the IL-18 gene, IL18-607A/C, and -137C/G were determined by analysis of a “real-time PCR/Melting curve”. Results Serum creatinine, cystatin C, CRP, IL-18, TOS, and OSI levels significantly decreased after transplantation. Post-transplant levels of serum TAC and estimated GFR demonstrated consistent significant increases. Serum IL-18 levels were significantly higher in patients with IL-18-137 GG and IL-18-607 CC genotypes before transplantation. Conclusion Our results indicate that the IL-18-137 GG and -607 CC genotypes contribute to higher IL-18 levels; however, the influence of these polymorphisms on oxidative stress has not been observed.
Introduction
Interleukin (IL-18) is a macrophage-derived multifunctional cytokine involved in both mediator and predictor of acute renal injury; it mediates a wide range of inflammatory and oxidative responses including renal injury, fibrosis, and graft rejection. IL-18 might be a “connecting” molecule between inflammation and fibrosis. IL-18 plays a role in the development of oxidative stress; studies using inflammasome NLRP3/NALP3, known to induce the expression of IL-18, demonstrated increased production of reactive oxygen species.Citation1 The NLRP3 inflammasome, one of the best characterized, controls the activation of caspase-1. Following activation, cleaved caspase-1 will cleave pro-IL-1β and pro-IL-18 into biologically active pro-inflammatory cytokines – mature IL-1β and mature IL-18, which are then released into the extracellular environment.Citation2
Oxidative stress, defined as an excess of pro-oxidant status not balanced by an adequate antioxidant defense system, can induce inflammation by activating nuclear factor-kappa B (NF-κB) and stimulating the subsequent production of proinflammatory cytokines and chemokines.Citation3,Citation4 Markers of oxidative stress, including elevated levels of malondialdehyde (MDA) and reduced antioxidant activity, have been reported in renal patients.Citation5–9 The normalization of kidney function after transplantation can improve oxidative stressCitation10 but some reports suggested thatCitation11,Citation12 increased oxidative stress in renal transplant recipients, particularly in the early phaseCitation13,Citation14 and, thereafter, accompanied by chronic allograft dysfunction.Citation11,Citation15–18 Serum (or plasma) concentrations of different oxidant and antioxidant molecules can be measured in laboratories separately; however, the measurements are time-consuming, labor-intensive, costly, and require complicated techniques. Since the measurement of different oxidant/antioxidant molecules separately is not practical, the measurements of these molecules as total oxidant status (TOS) and total antioxidant capacity (TAC) are more applicable.Citation19
It has been well recognized that the promoter polymorphisms of interleukin-18 (IL-18) influence the level of cytokine expression. Five different single nucleotide polymorphic positions in the promoter region have been identified: −656 G/T, −607 C/A, −137 G/C, +113 T/G, and +127 C/T; however, only two of them have functional relevance; a change from C to A at position −607 (rs1946518) and a substitution at position −137 from G to C (rs187238). It has been reported that the promoter -607 and -137 polymorphisms of IL-18 influence the level of cytokine IL-18 expression. Individuals homozygous for the −607 C and −137 G express higher levels of IL-18.Citation20
There are limited data related with oxidative stress and renal transplantation in the early stages. Therefore, we investigated the time-dependent changes in the oxidant/antioxidant balance during the first month after transplantation by measuring the TOS and TAC. We also determined the effect of IL-18 gene promoter polymorphisms on oxidative stress in renal transplant patients and examined the relationship between these markers and early graft function.
Materials and methods
Seventy-five living-donor kidney transplant recipients who were at least 18 years old were recruited into the study (28 female, 47 male; mean age: 38.28 ± 13.03). The study was conducted in accordance with the ethical standards of our Institutional Ethics Committee and with the Helsinki Declaration, and all patients provided written informed consent. Patients were excluded who had transplanted from a cadaveric donor, malignancy, combined (pancreas or liver) transplantation, or graft failure related to surgical causes. Demographic information about patients such as age, sex, HLA compatibility, primary disease, and dialysis duration are presented in .
Table 1. Demographic features of all patients and IL18–607/–137 gene polymorphism groups.
As immunosuppressive treatment protocol, calcineurin inhibitors (Tacrolimus, an initial dose of 1.5 mg/kg/day-two equal doses), mycophenolate mofetil (2 × 1 g/d), monoclonal antibodies (basiliximab 20 mg, 0 and 4th day), mTOR inhibitors (Everolimus, 2 × 1 mg starting dose in two equal doses) was used. Drug doses were adjusted according to the target blood levels.
The blood samples were collected immediately before and after surgery at day 7, month 1. The samples were centrifuged at 4000 rpm for 5 min. The sera were stored until analysis day at −80 °C. Serum creatinine and CRP measurements were performed by using original commercial kits in the Cobas 8000 auto analyzer (Roche Diagnostics, Mannheim, Germany). The results were expressed as mg/dL. Serum cystatin C levels were measured by using the particle-enhanced immunonephelometric (PENIA) method in a BN II Nephelometer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). The results were given as mg/L. Serum IL-18 levels were measured by Enzyme-Linked Immunosorbent Assay (ELISA) method (eBioscience Human IL-18 Instant ELISA, eBioscience Inc., San Diego, CA) (catalog no. BMS267INST). The results are expressed as pg/mL. Estimated glomerular filtration rates (eGFR) were calculated using the “Chronic Kidney Disease Epidemiology” (CKD-EPI) formula.Citation21
Serum TAC levels were determined using an automated measurement method as previously described by Erel.Citation19 In the assay, ferrous ion solution, present in reagent 1, was mixed with hydrogen peroxide, present in reagent 2. The sequentially produced radicals, such as brown-colored dianisidine radical cation produced by the hydroxyl radical, are also potent radicals. Using this method, the antioxidative effect of the sample on the potent free radical reactions initiated by the produced hydroxyl radical was determined. The results were expressed as mmol Trolox Equiv/L.
Serum TOS levels were determined by using an automated measurement method developed by Erel.Citation19 Oxidants present in the sample oxidize the ferrous ion–o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of μmol H2O2 Equiv/L. Oxidative stress index (OSI) values were calculated using a TOS/TAC ratio. The results were given as arbitrary units.
Genomic DNA extracts are obtained from peripheral venous blood by using DNA Isolation Kit I (automatic Isolation Kit) at Magna Pure LC instrument (Roche Diagnostics, Mannheim, Germany) for IL-18 gene promoter -607 A/C and -137 C/G polymorphism analysis. Real-time PCR/melting curve genotyping analysis was performed by using Roche Light Cycler 2.0 device (Roche Diagnostics, Mannheim, Germany) with FRET (Fluorescence Resonance Energy Transfer) hybridization probes (TIB MOLBIOL, Berlin, Germany, catalog no. for IL-18-137 SNP, for rs187238IL18 and IL-18-607 SNP; rs1946518IL18). Polymerase chain reactions were performed in a total reaction volume of 20 μL.
All statistical analyses were done with SPSS version 20.0 (SPSS Inc., Chicago, IL) and a significance level of 0.05 was considered. A Kolmogorov–Smirnov test was performed to assess the deviation from a normal distribution. Quantitative variables were summarized as mean and standard deviation (SD), or as median. Differences among all parameters at different time points were assessed by ANOVA, Student t test, or Mann–Whitney's U test for quantitative variables. The analysis of correlation was done by Spearman or Pearson test.
Results
The Turkish transplant patient population consists wholly of patients of Caucasian descent. General patient characteristics and demographic features are shown in . Genotypes for IL-18-607 promoter region polymorphism are defined as homozygous-Wild (CC), homozygous-mutant (AA), and heterozygous (CA). Similarly IL-18-137 promoter polymorphism is defined as homozygous-Wild (GG), homozygous-mutant (CC), and heterozygous (GC). The distribution of alleles and genotypes of the analyzed cytokine polymorphisms is presented in . The frequency of the genotype IL-18-607 CC was 53%, whereas 30% of patients were CA and 17% of patients were AA. The frequency of the genotype IL-18-137 GG was 65%, whereas 32% of patients were GC and 3% of patients were CC.
Table 2. Frequency of alleles and genotypes of interleukin-18 gene promoter polymorphism in kidney recipients.
summarizes the serum creatinine, cystatin-C, CRP, IL-18, TOS, TAC levels, OSI, and eGFR values in patients before and after transplantation at day 7 and month 1. Serum creatinine and cystatin C levels decreased after transplantation, while eGFR values increased. No significant differences were observed between post-transplant day 7 and month 1 for these parameters (). Serum IL-18 levels were significantly decreased after transplantation. Serum CRP levels were found to have significantly decreased at month 1. Serum TOS levels and OSI values were significantly higher before transplantation, while serum TAC levels were found to be significantly lower (). We did not observe any significant differences between post-transplant day 7 and month 1 for oxidative stress parameters.
Figure 1. The oxidative stress parameters before and after transplantation at day 7 and month 1. Data are given as mean ± standard error.
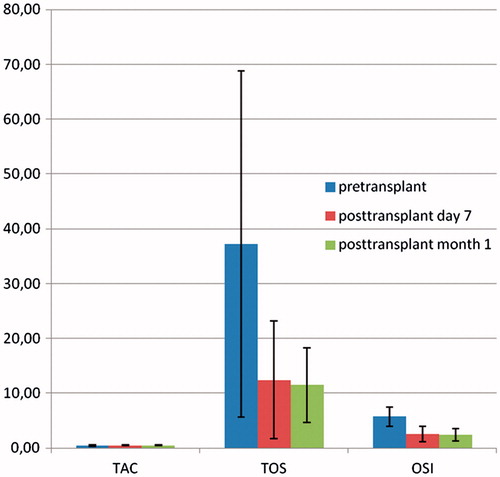
Table 3. Serum creatinine, cystatin-C, CRP, IL-18, TAC, TOS levels, OSI, and eGFR values before and after transplantation at day 7 and month 1. Data are given as mean ± standard error.
We observed significantly negative correlations between TAC with TOS and OSI before transplantation (respectively, r = −0.729, r = −0.938, p = 0.001). A significantly strong positive correlation was also found between TOS and OSI in same period (r = 0.878, p = 0.001). While we found significantly negative correlations between TAC with TOS and OSI at day 7 after transplantation (respectively, r = −0.673, p = 0.001, r = −0.706, p = 0.010), a strong positive correlation was found between TOS and OSI (r = 0.978, p = 0.001). Similar findings were also observed at month 1 after transplantation (for TAC vs. TOS and OSI; respectively, r = −0.512, p = 0.001, r = −0.613, p = 0.001; for TOS and OSI; r = 0.969, p = 0.001).
Patients with the IL-18-137 GG and IL-18-607 CC genotype had significantly higher IL-18 serum levels compared with other genotypes. Although we observed significant differences in serum IL-18 levels according to IL-18 607 and -137 polymorphisms, the influence of these polymorphisms on oxidative stress parameters have not been observed. Due to the low number of IL-18-137 CC mutant patients (n = 2), these data were not include in the study.
Discussion
On one hand, dialysis therapies are able to improve the metabolic status in patients with end-stage renal disease (ESRD). On the other hand, they have an ameliorating effect on inflammation and oxidative stress. Regarding renal transplantation, the best option in the renal replacement therapies is associated with significantly improved long-term survival. Several theories have been proposed for this survival advantage including better clearance of uremic toxins, improvement of cardiac capacity, and nutritional status. The normalization of kidney function after transplantation can improve oxidative stress, but some reports suggested that increased oxidative stress in renal transplant recipients, particularly in the early phase and thereafter, accompanied by chronic allograft dysfunction.Citation10–18,Citation22
In this study, we investigated the time-dependent changes in oxidative stress and inflammation with restoration of renal function by transplantation. Our results demonstrate a rapid decrease in serum TOS, IL-18, CRP levels, and OSI values after renal transplantation. Serum TAC levels and eGFR values demonstrated consistent increases after transplantation. We observed that restoration of renal function by transplantation improves the chronic inflammation and oxidative stress associated with uremia. This improvement is a consequence of reactive oxygen species (ROS) detoxification by the renal tubular function.Citation23,Citation24 Studies show that renal tubular epithel arranges the redox status of ultrafiltrate in selective transport and metabolic functions. The aldehyde dehydrogenase enzyme is highly active in the renal cortex, and loss of this important renal detoxifying mechanism is primarily the cause of ROS accumulation in ESRD.Citation10
Calcineurin inhibitors (cyclosporine and tacrolimus) are the main group of drugs necessary to prevent graft rejection, and it is possible that these therapies may also increase inflammation and oxidative stress. It has been recorded in some studies that cyclosporine causes enhancement in production of reactive oxygen species in kidney and heart transplant recipients. In another study, the effects of calcineurin inhibitors on total oxidant and antioxidant status were compared and no significant differences were found.Citation25 In our study, the effect of different immunosuppressive drugs on serum oxidant and antioxidant parameters was not assessed, because all patients were on a therapy with tacrolimus. We observed that oxidative stress significantly decreased in our patients under tacrolimus therapy.
Oxidative stress is also involved as one of the most important components of the ischemia–reperfusion (I/R) process and renal injury in renal transplantation.Citation26–28 When the kidney scavenging capacity is insufficient for the balancing of oxidative stress, increased production of ROS might trigger an inflammatory response within the graft. These processes lead to tissue damage and graft dysfunction. On one hand, Fonseca et al.Citation5 reported that MDA levels significantly decreased during the first post-transplant week when TAS levels did not exhibit any significant changes even though they classified the patients according to delayed graft function. On the other hand, we observed significant differences for TOS and TAC levels before and after transplantation. In contrast to their findings, our study highlights that the total antioxidant status enhanced while TOS levels decreased with transplantation.
The nuclear factor E2 (NFE2)-related factor 2 (Nrf2) protein that regulates the expression of antioxidants protects against oxidative damage triggered by injury and inflammation. Disruption of Nrf2 has been reported to cause increased production of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 in experimental mouse models. Williams et al.Citation29 reported that the increased oxidative stress correlated with increased IL-18 expression in mice deficient in NFE2-Nrf 2. Recent studies have also demonstrated that IL-18 levels are associated with the severity of renal damage. It has been shown that the individual cytokine polymorphisms play an important role in graft function.Citation20,30 Therefore, we investigated the time-dependent changes in the oxidant and the antioxidant balance during the first month after transplantation and examined the effect of IL-18 gene promoter polymorphisms on oxidative stress. We observed significant differences in serum IL-18 levels according to IL-18-607 and -137 polymorphisms. Despite improved kidney function and lower inflammatory and oxidative stress biomarkers in the early post transplant period, the influence of IL-18-607 and -137 polymorphisms on these markers have not been observed.
Although the results presented in our study are interesting, some potential limitations should be considered. Due to the limited number of patient, differences of IL-18 gene polymorphisms in various populations, and lengthy monitoring needs of patients, additional larger studies are required to further confirm the association of cytokine gene polymorphisms with oxidative stress.
Disclosure statement
The authors report that they have no conflicts of interest. Funding information
In this study, Akdeniz University Scientific Research Management Unit is supported by 2012.04.0103.003 with Project No.
References
- Tsai P, Ka S, Chang J, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51:744–754.
- Fann DY, Lee S, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 2013;12:941–966.
- Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007;71:167–172.
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667.
- Fonseca I, Reguengo H, Almeida M, et al. Oxidative stress in kidney transplantation: malondialdehyde is an early predictive marker of graft dysfunction. Transplantation. 2014;97:1058–1065.
- Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016.
- Miguel A, Miguel A, Linares M, et al. Evidence of an increased susceptibility to lipid peroxidation in red blood cells of chronic renal failure patients. Nephron. 1988;50:64–65.
- Mimić-Oka J, Simić T, Djukanović L, Reljić Z, Davicević Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin Nephrol. 1999;51:233
- Paul JL, Sall ND, Soni T, et al. Lipid peroxidation abnormalities in hemodialyzed patients. Nephron. 1993;64:106–109.
- Simmons EM, Langone A, Sezer MT, et al. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in endstage renal disease patients. Transplantation. 2005;79:914–919.
- Simic-Ogrizovic S, Simic T, Reljic Z, et al. Markers of oxidative stress after renal transplantation. Transpl Int. 1998;11:125–129.
- Campise M, Bamonti F, Novembrino C, et al. Oxidative stress in kidney transplant patients. Transplantation. 2003;76:1474–1478.
- Ardalan MR, Estakhri R, Hajipour B, et al. Erythropoietin ameliorates oxidative stress and tissue injury following renal ischemia/reperfusion in rat kidney and lung. Med Princ Pract. 2013;22:70–74.
- Zahmatkesh M, Kadkhodaee M, Mahdavi-Mazdeh M, et al. Oxidative stress status in renal transplant recipients. Exp Clin Transplant. 2010;8:38–44.
- Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol. 2007;293:F445
- Cristol JP, Vela C, Maggi MF, Descomps B, Mourad G. Oxidative stress and lipid abnormalities in renal transplant recipients with or without chronic rejection. Transplantation. 1998;65:1322–1328.
- Raj DS, Lim G, Levi M, Qualls C, Jain SK. Advanced glycation end products and oxidative stress are increased in chronic allograft nephropathy. Am J Kidney Dis. 2004;43:154–160.
- Vos IH, Joles JA, Rabelink TJ. The role of nitric oxide in renal transplantation. Semin Nephrol. 2004;24:379.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119.
- Kolesar L, Novota P, Krasna E, et al. Tissue Antigens: Polymorphism of interleukin-18 promoter influences the onset of kidney graft function after transplantation. Tissue Antigens. 2007;70:363–368.
- Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495.
- Dubourg L, Michoudet C, Cochat P, Baverel G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J Am Soc Nephrol. 2001;12:1615–1623.
- Michoudet C, Baverel G. Metabolism of acetaldehyde in human and baboon renal cortex. Ethanol synthesis by isolated baboon kidney-cortex tubules. FEBS Lett. 1987;216:113–117.
- Akbasli AC, Keven K, Erbay B, Nebioglu S. Changes in oxidative stress in renal graft patients receiving calcineurin inhibitors: Cyclosporine versus tacrolimus. Exp Clin Transplant. 2012;10:439–445.
- Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:461–470.
- Hariharan N, Zhai P, Sadoshima J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal. 2011;14:2179–2190.
- Dolegowska B, Blogowski W, Domanski L. Association between the perioperative antioxidative ability of platelets and early post-transplant function of kidney allografts: A pilot study. PLoS One. 2012;7:29779–29786.
- Williams MA, Rangasamy T, Bauer SM, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559.
- Kim C, Ryu H, Choi J, et al. Association of G-137C IL-18 promoter polymorphism with acute allograft rejection in renal transplant recipients. Transplantation. 2008;86:1610–1614.
