Abstract
Objective The objective of this study is to explore the efficacy and safety of mizoribine (MZR) in treating nephrotic syndrome patients afflicted with hepatitis B virus (HBV). Methods The present study included 36 nephrotic syndrome patients accompanied with HBV infection. A draft of MZR (150–200 mg/d), methylprednisolone (0.6–0.8 mg/kg·d), and entecavir (0.5 mg/d) was administered to study patients over 24 weeks. The serum albumin (AlB), 24-h urine protein (24-U-TP), liver and renal functions, and HBV-DNA were quantified before and at 2, 4, 8, 12, 16, 20, and 24 weeks after the treatment. The adverse responses were recorded. Results The AlB levels of patients increased gradually after comprehensive treatment, while the 24-U-TP, serum cholesterol, and triglyceride (TG) levels declined gradually. The changes at 24 weeks post-treatment were statistically significant. Compared with the levels before treatment, the HBV-DNA, transaminase, and renal functions of the patients were not significantly altered after the treatment. No evident adverse response was found. Conclusion Treatment using MZR in combination with methylprednisolone and entecavir in HBV-positive nephrotic syndrome patients displays significant efficacy with a low incidence of adverse reactions.
Introduction
Studies have shown that more than 0.1 billion people in China are infected with hepatitis B virus (HBV), including 25% afflicted with chronic hepatitis B.Citation1 Due to the high HBV infection rate, the incidence of nephrotic syndrome in patients positive for serum HBV markers is very common. The treatment of nephrotic syndrome mainly requires the combined use of glucocorticoids and immunosuppressants. However, the combination of these two drugs increases the replication of HBV, aggravates the symptoms of hepatitis B infection, and even induces life-threatening fulminant hepatitis. Safe and effective immunosuppressants for the treatment of nephrotic syndrome in patients with HBV infection are under development. In recent years, 36 HBV-positive nephrotic syndrome patients were treated in our department with mizoribine (MZR), a new immunosuppressant, according to the pathological types, and the biochemical parameters. The clinical efficacy of MZR was compared before and after the intervention. This study provides new evidence for the treatment of HBV-positive nephrotic syndrome.
Patients and methods
Ethics statement
This study was approved by the Ethics Committee of the Third Xiangya Hospital, Central South University, Hunan, China, on 1 March 2012. Renal biopsy was obtained from cases with tissue following diagnosis. All individuals signed written informed consent before research.
General characteristics
Thirty-six patients (24 males and 12 females) confirmed with nephrotic syndrome and HBV infection by needle biopsy of kidney between July 2012 and December 2013 were included. The patients’ median age was 31 years (19–52 years), with a disease duration ranging from 1 to 8.5 months. The renal pathology of these 36 patients included two with minimal change nephropathy (MCGN), eight with mild to moderate mesangial proliferative glomerulonephritis (MsPGN), six with membranous nephropathy (MN), eight with focal segmental glomerulosclerosis (FSGS), and 12 with IgA nephropathy (IgAN) (Lee stage II: three patients; stage III: seven patients; stage IV: one patient; and stage V: one patient) ().
Table 1. Clinical remission rates in patients with different renal pathologies (n).
Grouping: All the included patients met the diagnostic criteria of nephrotic syndrome and showed positive results of HBV antigen (including patients with HBV-associated nephropathy, and idiopathic nephrotic syndrome accompanied with HBV infection). The patients with diabetic nephropathy, lupus nephritis, and Henoch–Schönlein purpura nephritis were excluded. The treatment regimen for the patients comprised entecavir, and glucocorticoids plus MZR. The patients were followed up for 6 months.
Measurement methods
Renal needle biopsy was performed using Tur-Cut needle under the guidance of color ultrasonography. Three biopsies were obtained for immunofluorescent assay, light microscopy, and electron microscopic detection. The parameters measured in the immunofluorescent assay included HBs-Ag, HBc-Ag, and HBe-Ag. PCR was used to measure the HBV-DNA.
Drug therapy regimen
Entecavir (0.5 mg qd, Sino-American Shanghai Squibb Pharmaceuticals Ltd., Shanghai, China) was used to treat HBV infection in all the patients; anticoagulant and anti-platelet coagulation therapies were also performed. The patients with low serum albumin (AlB) during the treatment were supplemented with AlB. Patients with elevated liver enzymes were subjected to concurrent liver protection and enzyme-lowing therapies. In the event of increased HBV-DNA titer, the use of immunosuppressants was stopped immediately. Other treatments used included anti-hypertensive therapy, microcirculation improvement, and other symptomatic and supportive interventions. Detailed treatment regimen included methylprednisolone plus MZR. The initial dose of methylprednisolone was 0.6–0.8 mg/kg·d; the highest dose was 48 mg/d, which was tapered after 6–8 weeks. A draft of MZR (50 mg/tablet, Agms pharmaceutical Co., LTD, Tokyo Japan) was administered at a dose of 150–200 mg/d. The patients were followed up for 24 weeks.
Parameters
The parameters measured included serum AlB, alanine transaminase (ALT), blood urea nitrogen (BUN), serum creatinine (SCr), cholesterol (Chol), triglyceride (TG), 24-h urine protein (24-U-TP), and HBV-DNA.
Treatment efficacy
Complete remission (CR): CR was defined by complete remission of the clinical symptoms, and substantially decreased proteinuria, with 24-U-TP ≤ 0.3 g, serum AlB ≥ 35 g/L, normal liver and renal functions, and HBV-DNA titer less than 1 × 105/L.
Partial remission (PR): It is defined by PR of clinical symptoms, decreased proteinuria, 24-U-TP decrease by 50% of the baseline level to below 3.5 g, serum AlB 30 g/L or higher, normal liver and renal functions, and HBV-DNA titer less than 1 × 105/L.
Ineffective: Lack of efficacy is characterized by failure to improve clinical and laboratory parameters substantially, and less than 50% decrease in 24-U-TP compared with the baseline level.
Statistical analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL) was used for the statistical analysis. Quantitative data were described as means and standard deviations. Student’s t-test (for data with equal variances) or corrected t-test (for data with unequal variances) was used for the comparison of paired data. Chi-square test was used for the comparison of qualitative data. p < 0.05 was considered statistically significant.
Results
Efficacy evaluation
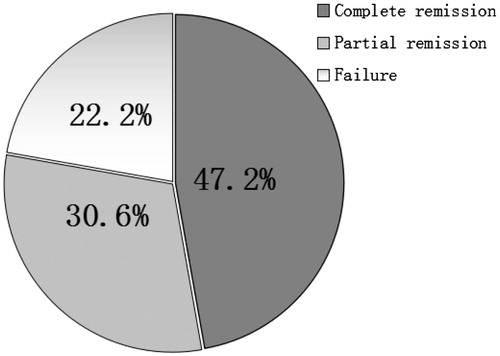
At the end of the treatment, CR, PR, and treatment failure were observed in 17, 11, and eight patients, respectively. The CR, PR, and the overall effective rates were 47.2%, 30.6%, and 77.8%, respectively ().
Laboratory investigation
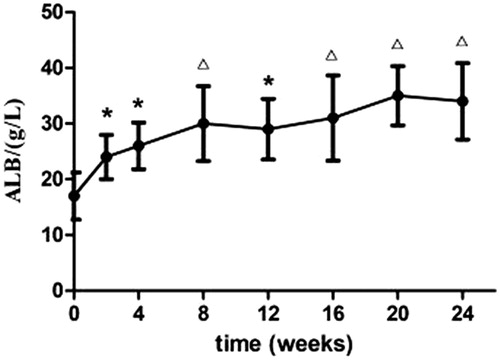
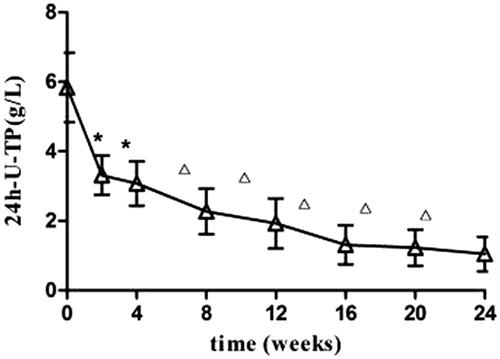
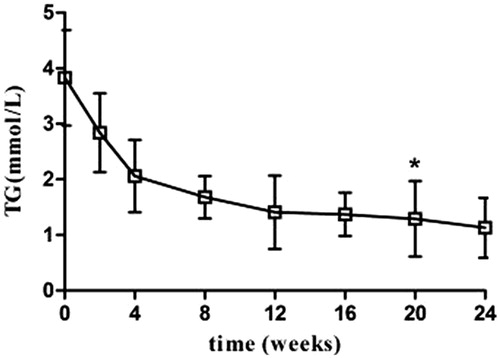
Partial remission was found in patients following 2 weeks of treatment. PR was characterized by increased urine volume, 24-U-TP decrease, and increased serum AlB (p < 0.05). The efficacy was more evident at 8 weeks of treatment, marked by disappearance of swelling, significant 24-U-TP decrease (p < 0.05), and significant serum AlB increase (p < 0.01). The liver and renal functions were not significantly altered after treatment compared with the levels before (p > 0.05) (and ).
Figure 4. Mizoribine therapy: serum cholesterol levels in patients with HBV-positive nephrotic syndrome.
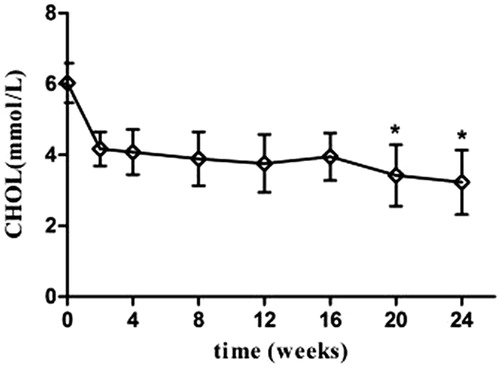
Table 2. Efficacy of mizoribine in treating nephrotic syndrome plus HBV infection.
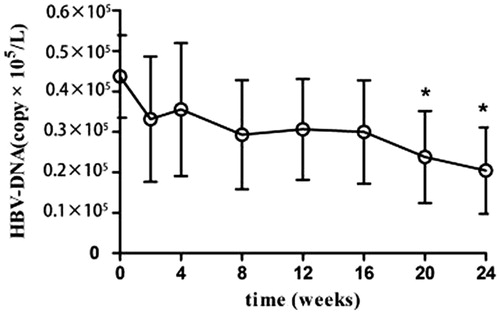
HBV–DNA titer
The HBV–DNA titer of all the 36 patients was less than 1 × 105/L before the treatment. After the treatment, the decrease in HBV-DNA levels varied, but was below 1 × 105/L ().
Adverse responses
No severe adverse response occurred in any of the 36 patients during the treatment. Three patients showed mild gastrointestinal reactions including loss of appetite and diarrhea. No immunosuppressant-related toxicity including abnormal renal function or bone marrow suppression was found.
Discussion
More than 0.1 billion Chinese are diagnosed with HBV infection.Citation1 Nephrotic syndrome accompanied with HBV infection is very common in clinical practices. Entecavir and adefovir dipivoxil are generally used to treat HBV. Glucocorticoids and immunosuppressants are also used to improve mass proteinuria and severe hypoproteinemia. Tacrolimus and mycophenolate mofetil are the commonly used immunosuppressants, which effectively improve the patients’ symptoms in nephrotic syndrome. However, severe infection results in fatal injuries and enormous economic burden.Citation2,Citation3 Leflunomide and azathioprine exhibit liver toxicity, and, therefore, are contraindicated for the treatment of HBV-positive nephrotic syndrome.Citation3,Citation4 MZR is an imidazole nucleoside that specifically inhibits the division and proliferation of T and B cells, resulting in humoral and cellular immune suppression.Citation5–7 As a new immunosuppressant, MZR is devoid of tumorigenicity and gonadal suppression effects, and with low bone marrow inhibition and hepatotoxic effects. Currently, MZR is used for the treatment of renal diseases including IgA nephropathy, nephrotic syndrome, lupus nephritis, and purpura nephritis, and is safe, reliable, and with acceptable efficacy.Citation8–17 As MZR increases the transcription activities of glucocorticoid receptors,Citation5,Citation18 increases the efficacy of glucocorticoids, and reduce, the viral replication induced by long-term use of high-dose glucocorticoids, the present study investigated the efficacy of MZR in combination with moderate dose of glucocorticoids in treating patients with HBV-positive nephrotic syndrome.
In the present clinical study, methylprednisolone, MZR, and entecavir were used as the combination treatment. The CR rate at the end of the treatment was 47.2%, and the overall effective rate was 77.8%. The efficacies of MZR were more pronounced in patients with MCD, mild to moderate MsPGN, MN, and IgAN with relatively low Lee stages. In contrast, patients with FSGS showed relatively poor efficacies. Overall, MZR exhibited rapid therapeutic effect, with obvious decrease in urine protein and clinical symptom improvement at 2 weeks of treatment, with a high overall remission rate. The liver and renal functions of patients were stable during the treatment, the HBV replication was well controlled, the incidence of adverse effects was low, and treatment compliance was good. However, the mechanisms involved in the efficacy of MZR in treating HBV-positive nephrotic syndrome are still unclear. We speculated that these mechanisms could be mediated via inhibition of abnormal mesangial cell proliferation, increased cellular ATP, enhanced levels of nephrin in glomerular epithelial cells, restoration of balanced intracellular energy levels, resulting in improvement in the morphology of glomerular basement membrane.Citation19–21 In addition, MZR inhibited the replication of hepatitis C virus RNA.Citation22 Additional studies are needed to investigate the effects of MZR on the replication of HBV DNA.
In summary, treatment using MZR in combination with methylprednisolone and entecavir improved the clinical remission rate and lowered the incidence of adverse responses in patients with HBV-positive nephrotic syndrome. As this study has no control, no other glomerular diseases and short period time for observing, multicenter, prospective, and randomized clinical trials are needed to further validate the findings of the present study.
Funding information
This project was supported by Science Foundation of the Health Department of Hunan Province (Grants no. 132013–029). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement
All authors have signed a Competing Interests and Acknowledgement Statement on submission of the manuscript.
References
- Bixin T, Jianjie C. Antiviral treatment status of Chronic hepatitis B. Chin J Compos Clin Med (in Chinese) 2004;6:128–132.
- Ekberg H, Bernasconi C, Nöldeke J, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant. 2010;25:2004–2010.
- Baek CH, Yang WS, Park KS, et al. Infectious risks and optimal strength of maintenance immunosuppressants in rituximab-treated kidney transplantation. Nephron Extra. 2012;2:66–75.
- Chertoff J, Alam S, Black M, et al. Azathioprine-induced hepatitis and cholestasis occurring 1 year after treatment. BMJ Case Rep. 2014;2014. doi: 10.1136/bcr-2014-206859.
- Kusmi T, Tsuda M, Katsumura T, et al. Dual inhibitory effect of bredinin. Cell Biochem Funct. 1989;7:201–204.
- Syokota S. Mizoribine: Mode of action and effects in clinical use. Pediatr Int. 2002;44:196–198.
- Kawasaki Y. Mizoribine: A new approach in the treatment of renal disease. Clin Dev Immunol. 2009;2009:684182.
- Shibasaki T, Koyama A, Hishida A, et al. A randomized open-label comparative study of conventional therapy versus mizoribine on lay therapy in patients with steroid-resistant nephrotic syndrome (postmarketing survey). Clin Exp Nephrol. 2004;8:117–126.
- Kawasaki Y, Hosoya M, Kobayashi S, et al. Oral mizoribine pulse therapy for patients with steroid-resistant and frequently relapsing steroid-dependent nephrotic syndrome. Nephrol Dial Transplant. 2005;20:2243–2247.
- Yunmra W, Suqanuma S, Uchida K, et al. Effects of long-term treatment with mizoribine in patients with proliferative lupus nephritis. Clin Nephrol. 2005;64:28–34.
- Kaneko K, Nagaoka R, Ohtomo Y, et al. Mizoribine for childhood IgA nephropathy. Nephron. 1999;83:376–377.
- Kawasaki Y, Hosoya M, Suzuki J, et al. Efficacy of multidrug therapy combined with mizoribine in children with diffuse IgA nephropathy in comparison with multidrug therapy without mizoribine and with methylprednisolone pulse therapy. Am J Nephrol. 2004;24:576–581.
- Xie YS, Huang SM, Wang L, et al. Efficacy and safety of mizoribine combined with losartan in the treatment of IgA nephropathy: A multicenter, randomized, controlled study. Am J Med Sci. 2011;341:367–372.
- Nagaoka R, Kaneko K, Yamashiro Y, et al. Mizoribine treatment for childhood IgA nephropathy. Pediatr Int. 2002;44:217–223.
- Hara S, Umino D, Someya T, et al. Protective effects of mizoribine on cyclosporine a nephropathy in rats. Pediatr Res. 2009;66:524–527.
- Ikezumi Y, Suzuki T, Karasawa T, et al. Contrasting effects of steroids and mizoribine on macrophage activation and glomerular lesions in rat thy-1 mesangial proliferative glomerulonephritis. Am J Nephrol. 2010;31:273–282.
- Matsumoto Y, Shimada Y, Nojima Y, et al. Efficacy of mizoribine followed by low-dose prednisone in patients with idiopathic membranous nephropathy and nephrotic-range proteinuria. Ren Fail. 2013;35:936–941.
- Tanaka H, Tsugawa K, Nakahata T, et al. Mizoribine pulse therapy for a pediatric patient with steroid-resistant nephrotic syndrome. Tohoku J Exp Med. 2005;205:87–91.
- Liu S, Xie Y, Lv Y, et al. A novel target of mizoribine inhibiting mesangial cell proliferation: S phase kinase-associated protein 2. Am J Nephrol. 2010;32:447–455.
- Fufang Q, Lei Y, Qiying F, et al. The inhibitory effect of mizoribine on mesangial cell proliferation in anti-Thy1 mesangial proliferative nephritis. J Pract Diagnosis Therapy (in Chinese) 2011;25:1185–1187.
- Nakajo A, Khoshnoodi J, Takenaka H, et al. Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol. 2007;18:2554–2564.
- Naka K, Ikeda M, Abe K, et al. Mizoribine inhibits hepatitis C virus RNA replication: Effect of combination with interferon-alpha. Biochem Biophys Res Commun. 2005;330:871–879.