Abstract
Objectives Propolis is a potent antioxidant and a free radical scavenger. Pharmacological induction of heat shock proteins (HSPs) has been investigated for restoring normal cellular function following an injury. In this study, effect of propolis on HSP-70 expression in methotrexate-induced nephrotoxicity and direct preventive effect of propolis in this toxicity were investigated. Material and methods A total of 40 male Wistar albino rats were divided into four groups: Group 1 was the untreated control. On the eighth day of the experiment, groups 2 and 3 received single intraperitoneal injections of methotrexate (MTX) at 20 mg/kg. Groups 3 and 4 received 100 mg/kg/day propolis (by oral gavage) for 15 d by the first day of the experimental protocol. Then the rats were decapitated under ketamine esthesia and their kidney tissues were removed. HSP-70 expression, apoptosis, and histopathological damage scores were then compared. Results MTX caused epithelial desquamation into the lumen of the tubules, dilatation, and congestion of the peritubular vessels and renal corpuscles with obscure Bowman’s space. The number of apoptotic cells (p = 0.000) and HSP-70 (p = 0.002) expression were increased in group 2. Propolis prevented the rise in number of apoptotic cells (p = 0.017), HSP-70 (p = 0.000) expression, and improved kidney morphology. Conclusions It was found that methotrexate gives rise to serious damage in the kidney and propolis is a potent antioxidant agent in preventing kidney injury.
Introduction
Propolis (bee glue) is the generic name given to resinous product of complex composition collected by honeybees from various plant sources.Citation1 It contains more than 300 components. Phenolic compounds, such as flavonoids, are major components and mainly responsible for the biological activity of propolis. It has been found to have many biological activities such as antimicrobial, immunomodulatory, anti-inflammatory, antioxidant, and anticarcinogenic.Citation2 It has been also reported to reduce toxic effect of some chemotherapeutic agents such as tamoxifenCitation3 and irinotecan. Nephrotoxic effect of irinotecan was reported to be reduced by propolis.Citation4
Methotrexate (MTX), a folic acid antagonist, is one of the most widely used anticancer drugs. It is used in the treatment of some malignant and autoimmune diseases. It has important toxic effects on many organs such as the kidney, liver, and bone marrow. More than 90% of MTX is cleared by the kidneys. Therefore, an impaired renal function by MTX delays its own elimination. Resulting sustained and elevated plasma concentration causes a marked enhancement of MTX’s other toxicities.Citation5 Thus, finding a nephroprotective agent is mandatory for the safe use of this important drug.
Heat-shock proteins (HSPs) are highly conserved and ubiquitously expressed molecular chaperones that help restore normal cellular function following an injury. Their expression is increased when there is a cellular stress such as thermal and ischemic injury.Citation6 There are efforts for inducing HSPs without the need for preceding cellular stresses. HSP-70 is one of the most frequently studied HSPs as a therapeutic target for cytoprotection.Citation7 The purpose of this study was to investigate the role of oxidative stress in MTX-associated kidney damage and also to show the probable protective effects of propolis against MTX-induced kidney damage at the histopathological and immunohistochemical levels.
Materials and methods
Sexually mature male, 8 weeks old, Wistar rats weighing 249.9 ± 23.18 g at the beginning of the experiment obtained from the Hakan Çetinsaya Experimental and Clinic Research Center, Erciyes University, Kayseri, Turkey, were used for this study. They were housed in plastic cages placed in a well-ventilated rat house and allowed ad libitum access to rat chow and water and were subjected to a natural photoperiod of 12-h light:dark cycle. This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Erciyes (Permit no. 13/83). The rats were randomly assigned to four groups of eight rats per group. Group 1 was the experimental control group and received 0.1 mL dimethyl sulfoxide (DMSO) by oral gavage. Group 2 received MTX. Group 3 was treated with both MTX and propolis. Group 4 was treated with propolis. Propolis was dissolved in DMSO. By the first day of the experimental protocol, groups 3 and 4 received 100 mg/kg/day propolis (by oral gavage) for 15 d. On the eighth day of the experiment, groups 2 and 3 received single intraperitoneal injections of MTX at 20 mg/kg (methotrexate DBL 500 mg/20 mL, Hospira UK Limites, Warwickshire, UK). The total duration of the experiment was 15 d. At the end of the experimental period, the animals were decapitated under ketamine (75 mg/kg) + xylazine (10 mg/kg) anesthesia and kidney tissue was removed.
Analysis of phenolic compounds in propolis by LC–MS/MS
The propolis was extracted using ethanol (96%) at room temperature for 1 week. To extract, 30 g propolis was dissolved with 100 mL ethanol. After extraction, the sample was filtered by paper filter and extract filtrated was evaporated to remove the residual solvent using a rotary evaporator at 40 °C. The dry propolis extract was diluted to 1000 mg/L in methanol and the mixture was filtrated with 0.2 μm microfiber filter prior to LC–MS/MS analysis.
LC–MS/MS analyses of the phenolic compounds of propolis were performed by using a Nexera model Shimadzu UHPLC coupled to a tandem MS instrument (Shimadzu Inc., Kyoto, Japan). The temperature of column (C18 reversed-phase Inertsil ODS-4) was fixed at 40 °C. The elution gradient consisted of mobile phase A (water, 5 mM ammonium formate and 0.1% formic acid) and mobile phase B (methanol, 5 mM ammonium formate and 0.1% formic acid). The flow rate of solvent was maintained at 0.5 mL/min and injection volume was 4 μL. MS detection was performed using Shimadzu LCMS 8040 model triple quadrupole mass spectrometer (Shimadzu Inc., Kyoto, Japan) equipped with an ESI source operating in both positive and negative ionization modes. LC–MS/MS data were collected and processed by LabSolutions software (Shimadzu, Kyoto, Japan). The multiple reaction monitoring (MRM) mode was used to quantify the analyses: the assay of the investigated compounds was performed following two or three transitions per compound, the first one for quantitative purposes and the second and/or the third one for confirmation.Citation8
Histopathology
Kidney tissue was removed, washed quickly with saline, and fixed with 4% (w/v) neutral formaldehyde (cat. no. 1039992500, Merck, Darmstadt, Germany) solution for 48 h, rinsed under running tap water for 24 h, followed by dehydration through a graded alcohol series. Tissues were made transparent in xylol and embedded in paraffin wax.
Immunohistochemistry
Tissue sections were deparaffinized, rehydrated, and then treated with 3% hydrogen peroxide to block endogenous peroxidase. After washing in phosphate-buffered saline (PBS), sections were treated with Lab Vision™ UltraVision™ Large Volume Detection System (TA-125-HDX, Thermo Fisher Scientific, Waltham, MA). HSP-70 (sc.33575; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 2.5 μg/mL in antibody diluents buffer (TA-125-ADQ, Thermo Fisher Scientific, Waltham, MA), then was applied overnight at 4 °C. The negative control omitted the primary antibody and overnight incubation with PBS. After further washes with PBS, the slides were incubated with biotin-conjugated secondary antibodies, followed by incubation with streptavidin-conjugated peroxidase working solution. Sections then were stained with 3,3′-p-diaminobenzidine tetrahydrochloride (TA-060-HDX, Thermo Fisher Scientific, and Waltham, MA), counterstained with Gill hematoxylin, dehydrated, and mounted. From each of the subjects, five different areas were evaluated in terms of the expression differences using the image J program.Citation9
Apoptosis (TUNEL)
The TUNEL method was utilized to show apoptosis of kidney tissue. An in situ Cell Death Detection Kit Fluorescein’ Kit (Roche, San Francisco, CA) was used. For the process, tissue sections deparaffinized and rehydrated. Having washed with PBS three times for 5 min, tissues were incubated with a TUNEL reaction mixture in a damp and dark place at 37 °C for 60 min. After washing with PBS three times for 5 min, tissues were contrast colored with 4,6-diamidine-2'-fenilindol. After covering the tissues with solution containing glycerol, they were all examined with the Olympus BX – 51 fluorescent microscope (Olympus Inc, Tokyo, Japan) at 450–500 nm wavelength. In order to estimate the apoptotic index, TUNEL-positive cells in 20 randomly chosen fields were counted. The apoptotic index was calculated as the percentage of TUNEL positive cells.Citation10
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (SPSS for windows, SPSS Inc, Chicago, IL, version 15.0). The Kolmogorov–Smirnov test was used to identify normal distribution of the data. In the case of normal distribution, quantitative variables were compared using one-way analysis of variance (ANOVA) and post hoc Tukey test. The non-parametric test (Kruskal–Wallis) was used for quantitative variables without normal distribution. These differences were considered significant when probability was less than 0.05.
Results
A phenolic compound of propolis extract is given in . As can be seen in , major compounds of propolis were trans-caffeic acid, vanillin, and chrysene. The amounts of trans-caffeic acid, vanillin, and chrysene were 168.91, 50.18, and 37.97 mg/g dry extract, respectively. Other major compounds were apigenin, naringenin, rhamnetin, quercetin, p-coumaric acid kaempferia, and hesperidin. However, the amounts of phenolics including quinic acid, malic acid, trans-aconitic acid, gallic acid, chlorogenic acid, tannic acid, rutin, hyperoxide, myristin, fisetin, 4-hydroxybenzoic acid, salicylic acid, hesperetin, and luteolin were determined as <1 mg/g dry extract.
Table 1. Phenolic compounds of propolis extract.
Histopathological findings
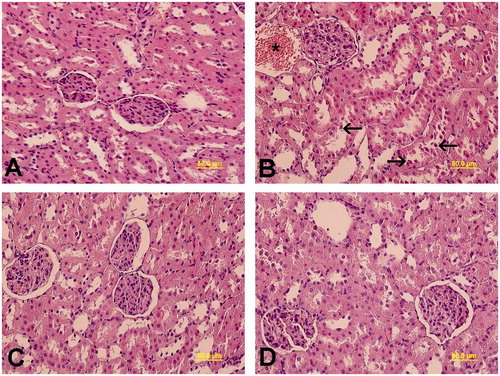
Light microscopic examinations exhibited normal renal corpuscles and tubules in the group 1 () and group 4 (). Epithelial desquamation into the lumen of the tubules, dilatation, and congestion of the peritubular vessels and renal corpuscles with obscure Bowman’s space in group 2 were observed (). Group 3 exhibited normal renal histology ().
Figure 1. Light microscopy of kidney tissue in different groups was observed. (A) In Group 1, normal kidney architecture was shown. (B) In group 2, epithelial desquamation into the lumen of the tubules (arrow), and dilatation and congestion of the peritubular vessels (*) were visualized. (C) In group 3, normal kidney architecture was observed. (D) In group 4, normal kidney architecture was exhibited. Kidney cross sections were stained with H&E.
Apoptotic findings
and illustrate apoptosis as demonstrated by TUNEL staining. The mean apoptotic index in the kidney of groups 1 and 2 was found to be 1.75 ± 0.38 and 9.85 ± 2.38, respectively. The increase in the apoptotic index was statistically significant in group 2 compared with the group 1 (p = 0.000). Group 3 resulted in the decrease of the number of TUNEL-positive cells and the apoptotic index was 2.86 ± 1.90. The decrease in the apoptotic index was statistically significant in group 3 compared with group 2 (p = 0.017). The apoptotic index in the kidney of group 4 was found to be 0.33 ± 0.79.
Figure 2. TUNEL staining of kidney tissue. TUNEL-positive cells (arrow) were mainly observed in distal tubule. (A) Group 1, (B) group 2, (C) group 3, and (D) group 4.
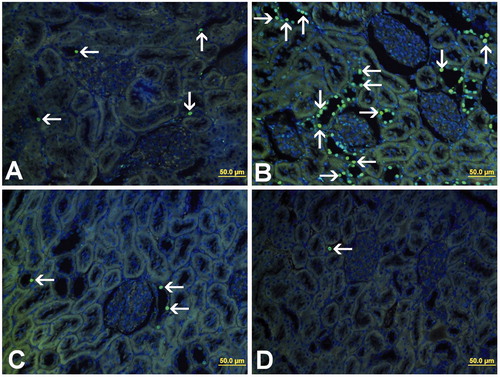
Table 2. Apoptotic index and HSP-70 expression of rats. Values are expressed as mean ± SE.
Immunohistochemical findings
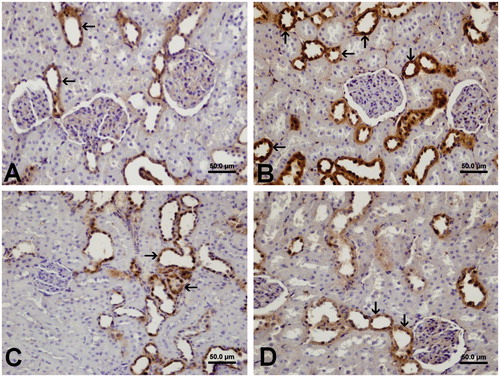
Immunohistochemical staining was performed using the avidin–biotin method to determine the kidney tissue expression of HSP-70. Expression of HSP-70 was observed in the distal tubules and collecting ducts in group 1 (). HSP-70 immunoreactivity was significantly greater (p = 0.002) in group 2 (133.24 ± 1.02) compared with group 1 (126.52 ± 1.77). HSP-70 expression was decreased (p = 0.000) in group 3 (124.06 ± 1.45) compared with the group 2. The HSP-70 expression in the kidney of group 4 (124.03 ± 1.79) was similar to that in the group 1.
Figure 3. Immunohistochemical localization of HSP-70 expression of the kidney tissue in the different groups. (A) Expression of HSP-70 was prominent in the distal tubules and collecting ducts in group 1. (B) Increased expression of HSP-70 was shown in the distal tubules and collecting ducts in group 2. (C) HSP-70 immunostaining was observed in the distal tubules and collecting ducts in group 3. (D) HSP-70 expression was shown in the distal tubules and collecting ducts in group 4.
Discussion
In this present study, on one hand, propolis restored kidney morphology disrupted by MTX. HSP-70 expression was increased as a response to MTX-induced nephrotoxicity. However, the amount of the expression returned to baseline levels with the improvement of nephrotoxicity by propolis. On the other hand, propolis did not affect HSP-70 expression in the normal kidney tissue.
There are several studies reporting protective effect of propolis against toxic effect of chemotherapeutic agents. In a study carried out in mice-bearing Ehrlich ascites tumors, propolis and its polyphenolic compounds protected blood, liver, and kidney cells from irinotecan toxicity.Citation4 In another study carried out in mice with mammary carcinoma, propolis prevented the epirubicin and radiation induced hematological toxicity.Citation11 In an ICR mouse model bearing a syngeneic Ehrlich ascites carcinoma, propolis significantly ameliorated the cytopenia induced by 5-fluorouracil and mitomycin C resulting in recovery of white as well as red blood cell counts.Citation12
MTX has potential side effects on many organs, particularly on the liver and kidney. The pathogenesis of MTX-induced nephrotoxicity is not clear. Precipitation of MTX and its metabolites in the renal tubules or direct toxic effect of MTX are believed to have role in the nephrotoxicity.Citation13 Hydration of the patient and alkalinization of urine does not prevent completely the renal damage,Citation14 suggesting that other mechanisms may also be involved in MTX-induced nephrotoxicity. Several studies report that MTX causes oxidative stress and this has role in its renal toxicity. MTX produces reactive oxygen species (ROS) and thus causes lipid peroxidation and leads to impairment in mitochondrial function.Citation15 Neutrophil infiltration and oxidative stress were suggested to cause MTX-induced nephrotoxicity in the rat.Citation16 Similarly, many nephrotoxic drugs including cisplatin and gentamicin are reported to damage the kidney by the production of ROS.Citation17 The ROS damage cellular macromolecules including membrane lipids, proteins, and nucleic acids, resulting in cellular damage. Several studies have confirmed the role of flavonoids in the deactivation of the free radicals.Citation18,Citation19 The effects of caffeic acid phenethyl ester (CAPE), an active flavonoid-like compound of propolis, on MTX-induced hepatotoxicity and nephrotoxicity were investigated by biochemical methods and histological examinations.Citation20 The authors reported that CAPE, with its free radical scavenging properties, prevented methotrexate-induced lipid peroxidation and neutrophil infiltration of the hepatic and renal tissues in the rat. In another study in Wistar albino rats, the nephrotoxic effect of MTX was associated with lipid peroxidation and reduction in antioxidant enzymatic defense capacity.Citation21 In this study, CAPE prevented nephrotoxicity and this effect was attributed to its scavenging activity for ROS in the kidney tissue. Therefore, we suggest that propolis prevents MTX-induced nephrotoxicity with its antioxidant properties. Supporting this hypothesis, many studies showed that different antioxidants such as vitamin C, b-carotene, and flavonoids reduce the adverse effects of the some chemotherapeutic agents.Citation11,Citation22
Apoptosis may have a role in renal dysfunction and is frequently observed in acute renal failure.Citation23 In this present study, MTX increased apoptosis and propolis prevented this increase. Similarly, in a study in rat kidney, authors showed increased apoptosis after MTX treatment.Citation24 Herman et al.Citation25 and Mazur et al.Citation26 showed that MTX causes apoptosis in T lymphocytes, human uterine cervix cancer, and the normal fibroblastic rat kidney. MTX is believed to induce apoptosis through oxidative stress that results in damage to DNA.Citation25 Supporting this hypothesis, apricot diet which contains polyphenols and flavonoids significantly decreased MTX-induced apoptosis in the rat.Citation24 Also, Vardi et al.Citation27 indicated that β-carotene exhibited a protective effect on MTX-induced apoptosis in testicular cells..
In this present study, HSP-70 expression was increased in nephrotoxic group. Marked increase in HSP-70 expression against ischemia-reperfusion injury was shown in the rat kidney.Citation28 Also, it was reported that HSP-70 knock-out mice had worse kidney function, tubular injury, and survival following renal ischemia–reperfusion injury.Citation29 On one hand, it is reported that HSPs interact with important proteins involved in apoptotic pathways and this has crucial consequences for cell survival, proliferation, and apoptosis following ischemia–reperfusion injury.Citation30 As a result, renal epithelial cells may be rescued from apoptotic cell death. On the other hand, propolis did not induce HSP-70 expression in this present study.
We have demonstrated the protective effect of propolis on MTX-induced kidney injury for the first time. The administration of propolis, a novel antioxidant, improved the histopathological parameters, increased immunoexpression of MTX-induced kidney HSP-70. Thus, antioxidants may be useful as pharmacological agents to protect against MTX-induced kidney injury.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–571.
- Lotfy M. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7:22–31.
- Albukhari AA, Gashlan HM, El-Beshbishy HA, Nagy AA, Abdel-Naim AB. Caffeic acid phenethyl ester protects against tamoxifen-induced hepatotoxicity in rats. Food Chem Toxicol. 2009;47:1689–1695.
- Oršolić N, Benković V, Lisičić D, Đikić D, Erhardt J, Knežević AH. Protective effects of propolis and related polyphenolic/flavonoid compounds against toxicity induced by irinotecan. Med Oncol. 2010;27:1346–1358.
- Stark AN, Jackson G, Carey PJ, Arfeen S, Proctor SJ. Severe renal toxicity due to intermediate-dose methotrexate. Cancer Chemotherapy Pharmacol. 1989;24:243–245.
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592.
- O'Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J. Heat-shock proteins and acute ischemic kidney injury. Nephron Exp Nephrol. 2014;126:167–174.
- Ertas A, Boga M, Yilmaz MA, et al. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymusnummularius (Anzer tea): Rosmarinic acid. Ind Crops Prod. 2015;67:336–345.
- Sonmez MF, Narin F, Akkus D, Turkmen AB. Melatonin and vitamin C ameliorate alcohol-induced oxidative stress and eNOS expression in rat kidney. Ren Fail. 2012;34:480–486.
- Bayatli F, Akkus D, Kilic E, Saraymen R, Sonmez MF. The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: An experimental study. World J Urol. 2013;31:615–622.
- Oršolić N, Bašić I. Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP). Biomed Pharmacother. 2005;59:561–570.
- Suzuki I, Hayashi I, Takaki T, Groveman DS, Fujimiya Y. Antitumor and anticytopenic effects of aqueous extracts of propolis in combination with chemotherapeutic agents. Cancer Biother Radiopharm. 2002;17:553–562.
- Messmann R, Allegra C. Antifolates. Cancer Chemotherapy and Biotherapy. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:139–184.
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703.
- Babiak RMV, Campello AP, Carnieri EG, Oliveira M, Benigna M. Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct. 1998;16:283–293.
- Kolli W, Abraham P, Isaac B, Selvakumar D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy. 2009;55:83–90.
- Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–353.
- Sadzuka Y, Sugiyama T, Shimoi K, Kinae N, Hirota S. Protective effect of flavonoids on doxorubicin-induced cardiotoxicity. Toxicol Lett. 1997;92:1–7.
- Mesbah L, Soraya B, Narimane S, Jean P. Protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid–peroxydation and increase of liver glutathione. Hematology. 2004;7:59–67.
- Çakır T, Özkan E, Dulundu E, et al. Caffeic acid phenethyl ester (CAPE) prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pharm Pharmacol. 2011;63:1566–1571.
- Öktem F, Yilmaz HR, Ozguner F, et al. Methotrexate-induced renal oxidative stress in rats: the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health. 2006;22:241–247.
- Borek C. Dietary antioxidants and human cancer. Integr Cancer Therap. 2004;3:333–341.
- Jo SK, Yun SY, Chang KH, et al. MSH decreases apoptosis in ischemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dialysis Transplant. 2001;16:1583–1591.
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J Physiol Biochem. 2013;69:371–381.
- Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res. 2005;54:273–280.
- Mazur AJ, Nowak D, Mannherz HG, Malicka-Błaszkiewicz M. Methotrexate induces apoptosis in CaSki and NRK cells and influences the organization of their actin cytoskeleton. Eur J Pharmacol. 2009;613:24–33.
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil Steril. 2009;92:2028–2033.
- Zhang PL, Lun M, Schworer CM, et al. Heat shock protein expression is highly sensitive to ischemia–reperfusion injury in rat kidneys. Ann Clin Lab Sci. 2008;38:57–64.
- Wang Z, Gall JM, Bonegio RG, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79:861–870.
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761.
