Abstract
Background: Hypertension is one of the important contributing factors linked with both causation and development of kidney disease. It is a multifactorial, polygenic, and complex disorder due to interaction of several risk genes with environmental factors. The present study was aimed to explore genetic polymorphism in ACE-1 gene as a risk factor for CKD among hypertensive patients. Methods: Three hundred patients were enrolled in the study. Ninety were hypertensive patients with CKD taken as cases, whereas 210 hypertensive patients without CKD were taken as controls. Demographic data including age, sex, Body mass index (BMI), and other risk factors were also recorded. DNA was extracted from blood by salting out method. Genotyping of ACE gene was done by PCR technique. All the statistical analysis was done by using Epi Info and SPSS version 16 software (SPSS Inc., Chicago, IL). Results: Mean age was higher in the control group (p < 0.05). Variables among two groups were compared out of which age, BMI, hemoglobin (Hb) was found to be statistically significant whereas other variables like systolic blood pressure, triglyceride and low-density lipoprotein were not. Blood urea and serum creatinine levels were statistically significant in the two genotypes (p < 0.05). Total and HDL cholesterol were statistically significant for DD genotype of ACE gene (OR = 1.42, 95% CI = 0.72–2.81). Similarly, the risk for CKD among hypertensive patients was also associated with D allele of ACE gene (OR = 1.25, 95% CI = 0.86–1.79). Conclusion: It is concluded that ACE-DD genotype may be a risk factor for the causation and development of chronic kidney failure among hypertensive patients.
Introduction
Hypertension is one of the common, polygenic, and complex disorder which is a consequences of interaction of several genes with each other along with environmental factors.Citation1,Citation2 It is more prevalent in those who have one or both hypertensive parents and many epidemiological studies suggest that genetic factors account for approximately 30% of the variation in blood pressure in various populations.Citation3,Citation4 The effect of genetic variability on the development of chronic kidney disease is clear. Although probability of development of chronic kidney disease (CKD) in a patient with a sibling having history of hypertension and CKD is very high but a clear mechanism is lacking. Better understanding of different phenotypes observed in end stage renal disease (ESRD) would permit us to determine whether a patient is genetically predisposed to such complications. Genetic variability in various genes affecting the pathogenesis in ESRD is hypothesized to contribute in a variable manner. Various genes from different components of Renin–Angiotensin System (RAS) are involved in the regulation of both blood pressure and renal function and might play a role in their interaction. The presence of insertion/deletion (I/D) polymorphism of Angiotensin-Converting Enzyme (ACE) affects the plasma and tissue ACE levels. DD genotype of ACE is associated with highest systemic and renal ACE levels, compared with the lowest ACE activity associated with carriers of II genotype. Rigat et al.Citation5 first reported that the ACE insertion (I)/deletion (D) polymorphism involves the presence or absence of a 287-bp sequence of DNA in intron 16 of the gene.
In the present study, we investigated whether genetic polymorphism in Angiotensin-Converting Enzyme-1 (ACE-1) gene lead to chronic kidney disease among hypertensive patients. Genotypic profiling was also compared in both hypertensive patients with or without CKD.
Materials and methods
Subjects
The study was conducted at Nephrology and Hypertension Clinic of our hospital. The study protocol was approved by the institutional ethics committee. Patients above 18 years of age with history of hypertension and on regular medication for last two years with or without CKD were included in the study. Patients having secondary hypertension preceding CKD, polycystic kidney disease and diabetes mellitus were excluded. Diagnosis of hypertension was established as per JNC-7 criteria (2003). A total of 300 patients were enrolled in the study. Ninety patients having history of hypertension and on regular treatment from last two years with CKD (stage 3 onwards i.e. eGFR <60 mL/min/1.73 m2) were taken as cases.Citation6 Out of 300 patients, 210 patients having hypertension without CKD (eGFR >60 mL/min/1.73 m2) were taken as controls. A written informed consent was obtained from all the patients and it was documented in the detailed performa. Necessary laboratory and biochemical parameters were carried out for all the patients.
Sample collection and analysis
Blood sample: Blood samples were collected from all the patients in the morning for measuring serum biochemical and lipid profile. Four milliliter of intravenous blood was collected from the patients into EDTA vacutainer under aseptic condition and stored at −4 °C for further extraction of genomic DNA.
DNA extraction: Genomic DNA was extracted from blood by salting out method as described by Miller et al.Citation7 and was purified by ethanol precipitation. Purified DNA was quantified by using a UV–VIS spectrophotometer. An absorbance of 1.8:2.0 or greater was considered and the final solution was stored at −4 °C. Purified DNA was further used as a template for ACE -I/D polymorphism analysis.
Determination of ACE genotype
PCR reaction was performed for detecting the ACE I/D polymorphism using the isolated DNA as template. A set of sequence specific primers as illustrated in previous studiesCitation8 were used to amplify the target DNA. PCR reactions were performed in a total volume of 25 μL per tube, containing 3 μL genomic DNA (50 ng), 0.5 μL MgCl2 (25 mM), 2.5 μL 10× DNA buffer, 0.5 μL of Taq DNA polymerase (1 units), 1 μL dNTPs (0.25 mM), 0.3 μL each of the following F5′-CTGGAGACCACTCCCATCCTTTCT-3′ and R5′GATGTGGCCATCACATTCGTCAGAT-3′ primers and 16.9 μL of deionized water. The DNA was amplified using standardized procedure given by Mondry et al.Citation8 with little modifications. In order to avoid the mistyping of ID genotype with DD, due to preferential amplification of the shorter D allele, a separate PCR was carried out in all the DD samples.
The PCR amplicon were analyzed by electrophoresis in 2% agarose gel and stained with ethidium bromide and photographed by using Gel doc imaging. The D and I allele size was carried out by using 50 bp–500 bp ΦHind III digest DNA ladder.
Statistical analysis
All the statistical analysis was done by using Epi Info and SPSS version 16 software (SPSS Inc., Chicago, IL). Qualitative data were represented by numbers and percentage. Chi-square test was used to observe the difference between the proportions. Odd ratio with 95% confidence interval was calculated to quantify the magnitude of the risk factors wherever required. Quantitative data was expressed by mean and standard deviation for normality distributed data. Normality of the data was observed by using the Kolmogorov–Smirnov normality test. t-Test was applied for observing the difference between the two mean for normally distributed data whereas Mann–Whitney’s U test was applied for non-normal data. p Value less than 0.05 was considered as significant.
Results
Demographic profile
Out of 90 cases, 48 (53.33%) were males and 42 (46.66%) were females. Among 210 patients in control group, 75 (35.71%) were males and 135 (64.28%) were females. Mean age reported in the present study was 43 ± 16 in case and 49.86 ± 11.07 in control groups. The mean BMI between the two groups was 21.11 ± 4.85 and 26.41 ±5.24, respectively. Mean diastolic blood pressure recordings were higher in cases as compared with the control group. Age, BMI, and DBP parameters were found to be statistically significant as shown in .
Table 1. Demographic profile of study patients.
Distribution of ACE genotype
The distribution of DD, ID, and II genotype in both the groups is shown in . The different band sizes of DD, ID, and II genotype are shown in . In the present study, polymorphism in DD genotype was associated with increased risk of developing CKD among hypertensive patients (OR = 1.19; 95% CI of 0.67–2.10). Similarly, higher risk was also found with the frequency of D allele (OR = 1.25; 95% CI = 0.86–1.79). The pooling effect of ID + II genotypes, when compared with DD genotype alone reveals that, hypertensive subjects with DD genotype are at more risk for CKD development (OR = 1.31; 95% CI = 0.75–2.30).
Figure 1. Representative photograph of ACE gene in HT patients with and without CKD. Lanes 1, 4, and 5: heterozygous ID samples (190 bp and 490 bp). Lanes 2 and 3: homozygous DD sample (190 bp). Lane 6: DNA ladder 50–500 bp (Hind III digest Ladder).
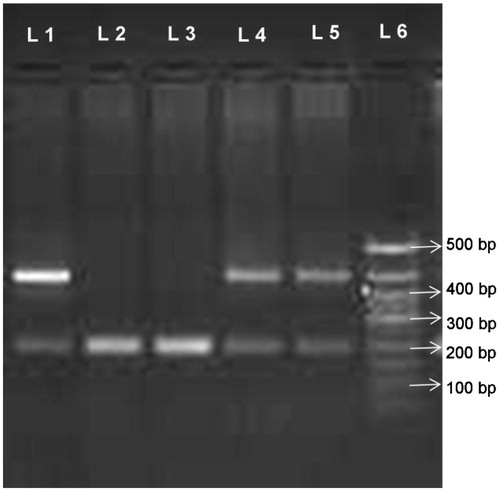
Table 2. Distribution of ACE genotype in the two groups.
Laboratory characteristics of patients with DD and non-DD genotype of ACE gene
Age, male/female ratio, BMI, triglyceride, and LDL cholesterol levels among all study patients bearing DD and non-DD genotypes of ACE gene polymorphism are shown in . Interestingly, higher levels of blood urea and serum creatinine, and lower levels of total and HDL cholesterol were reported in cases with non-DD polymorphism, which was found to be statistically significant (p < 0.05). In the control group, LDL cholesterol was the only parameter which showed statistical significance.
Table 3. Laboratory characteristics of patients with respect to ACE gene genotype.
Association of ACE-I/D genotype
A logistic regression analysis was used to adjust the odds ratio for cases and the ACE I/D genetic polymorphisms. The age, sex, and obesity emerged to be statistically significant between the two groups while micro-albuminuria was found to be a risk factor for the control group only. On comparison of DD genotype with non-DD genotype of ACE gene, results found were not statistically significant in both the groups.
Discussion
In the present study, we found that the development of chronic kidney disease is linked with DD genotype of ACE gene among hypertensive patients. ACE gene is one of the most intensely studied genes, because it plays a key role in the Renin–Angiotensin–Aldosterone System. The gene coding for ACE is subject to Insertion/Deletion polymorphisms. Many studies have addressed the role of ACE gene as a candidate gene responsible for cardiovascular and renal organ damage. Although, a few studies support a role of the D allele as a risk factor for target organ damage.Citation9–12 While, other studies have provided conflicting results.Citation13–15
The distribution of ACE-I gene polymorphism among cases was observed to be DD (34.44%), ID (37.78%), and II (27.78%). Frequency of D allele reported in cases was 53.35%. Similarly, the distribution of ACE gene genotype in control group was DD (28.57%), ID (38.57%), and II (32.86%). The frequency of D allele was 47.9% among control group patients. A trend was seen that DD polymorphism was present in more number of cases then controls. Tripathi et al.Citation16,Citation17 in their series of work on genetic polymorphisms, among ESRD patients, evaluated the genetic biomarkers related to post-dialysis patients and compared these biomarkers with healthy control subjects. In their study, D allele frequency was 50.5% and I allele was 49.5% among ESRD patients. But, in our study, all the CKD patients were hypertensive. Tripathi et al.Citation16,Citation17 have demonstrated that ACE-DD genotype may be a potential risk factor for development of chronic kidney disease. But, our study showed that subjects having DD genotype were at 1.42 times higher risk for the progression of chronic kidney disease as compared with II genotype, but it was not found statistically significant. In a study by Singh et al.Citation18, estimated and compared the prevalence of low Glomerular Filtration Rate (GFR), proteinuria and associated risk factors in North India using Cockcroft–Gault (CG) and Modification of Diet in Renal Disease (MDRD) equation. They found that unstandardized prevalence of low eGFR was 13.3% by CG equation and 4.2% by MDRD equation. In the present study, we have used Cockcroft–Gault equation for the estimation of GFR in both the groups.
When clinical parameters in cases were compared between DD and non-DD genotype, blood urea, and serum creatinine parameters were statistically significant. In lipid profile, two parameters HDL and total cholesterol were statistically significant for DD genotype. Similarly, other clinical parameters among the patients in the control group, LDL cholesterol was statistically significant for the DD genotype.
Our study did not show any association between DD polymorphism of ACE gene with essential hypertension in control group. Some studies haveCitation19–21 showed an association of DD polymorphism of ACE gene with essential hypertension, whereas other studies showed conflicting results.Citation22,Citation23 Patnaik et al.Citation24 conducted a study to see the association of ACE I/D polymorphisms with essential hypertension in the population of Odisha, an eastern Indian state. On comparing ACE gene polymorphisms in hypertensive patients with normotensive individuals, they concluded that ACE I/D polymorphisms were associated with essential hypertension among female patients in the study population. Similarly, another study by Srivastava et al.Citation25 investigated the association of I/D polymorphism of the ACE gene with essential hypertension in northern Indians. They compared ACE gene polymorphism of essential hypertension with healthy controls. They suggested that I allele of ACE I/D polymorphism is associated with essential hypertension in the study population.
Conclusion
In conclusion, we can say that ACE-DD genotype may be a risk factor for the chronic kidney disease among hypertensive patients. The risk is also linked with the frequency of D allele for the progression to chronic kidney disease. Further studies are required to understand the underlying mechanisms for progression to CKD and to prevent it in hypertensive patients.
Acknowledgements
We are thankful to Mrs. Renuka Saha (statistician), Department of Community Medicine, MAM College, New Delhi, for carrying out the statistical analysis in the present study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding information
The authors are thankful to UGC for providing financial support for this study.
References
- Basset el EA, Berthoux P, Cecillon S, et al. Hypertension after renal transplantation and polymorphism of genes involved in essential hypertension: ACE, AGT, AT1R and ecNOS. Clin Nephrol. 2002;57:192–2002.
- Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996;272:676–680.
- Levey AS. Clinical practice. Nondiabetic kidney disease. N Engl J Med. 2002;347:1505–1511.
- Sarkar T, Singh NP. Epidemiology and genetics of hypertension. JAPI. 2015;63:61–68.
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S47.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
- Mondry A, Loh M, Liu P, Zhu AL, Nagel M. Polymorphisms of the insertion / deletion ACE and M235T AGT genes and hypertension: Surprising new findings and meta-analysis of data. BMC Nephrol. 2005;6:1.
- Samani NJ, Thompson JR, O’Toole L, Channer K, Woods KL. A meta-analysis of the association of the deletion allele of the angiotensin converting enzyme gene with myocardial infarction. Circulation. 1996;94:708–712.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764.
- Broekroelofs J, Stegeman CA, Navis GJ, Tegzess AM, de Zeeuw D, de Jong PE. Risk factors for long-term renal survival after renal transplantation: A role for the angiotensin-converting enzyme insertion/deletion polymorphism? J Am Soc Nephrol. 1998;9:2075–2081.
- Hadjadj S, Belloum R, Bouhanick B, et al. Prognostic value of angiotensin-I converting enzyme I/D polymorphism for nephropathy in type 1 diabetes mellitus: A prospective study. J Am Soc Nephrol. 2001;12:541–549.
- Lindpainter K, Pfeffer MA, Kruetz R, et al. A prospective evaluation of an angiotensin-converting enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–711.
- Beige J, Schere S, Weber A, et al. Angiotensin converting enzyme genotype and renal allograft survival. J Am Soc Nephrol. 1997;8:1319–1323.
- Pei Y, Scholey J, Thai K, Suzuki M, Cattran D. Association of angiotensinogen gene T235 variant with progression of immunoglobin A nephropathy in Caucasian patients. J Clin Invest. 1997;100:814–820.
- Tripathi G, Dharmani P, Khan F, Sharma RK, Pandirikkal V, Agrawal S. High prevalence of ACE-DD genotype among North Indian end stage renal disease patients. BMC Nephrol. 2006;7:15.
- Tripathi G, Sharma RK, Baburaj VP, Sankhwar SN, Jafar T, Agrawal S. Genetic risk factors for renal failure among North Indian ESRD patients. Clin Biochem. 2008;41:525–531.
- Singh NP, Ingle GK, Saini VK, et al. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: An observational, cross-sectional study. BMC Nephrol. 2009;10:1–13.
- Chiang FT, Chern TH, Lai ZP, et al. Age-and gender-dependent association of the angiotensin-converting enzyme gene with essential hypertension in a Chinese population. J Hum Hypertens. 1996;10:823–826.
- Plasquale PD, Cannizzaro S, Paterna S. Does angiotensin-converting enzyme gene polymorphism affect blood pressure? Findings after 6 years of follow-up in healthy subjects. Eur J Heart Fail. 2004;6:11–16.
- Sameer AS, Syeed N, Tak SA, Bashir S, Nissara S, Siddiqi MA. ACE I/D polymorphism in hypertensive patients of Kashmiri population. Cardiol Res. 2010;1:1–7.
- Gupta S, Agrawal BK, Goel RJ, Sehajpal PK. Angiotensin-converting enzyme gene polymorphism in hypertensive rural population of Haryana, India. J Emerg Trauma Shock. 2009;2:150–154.
- Glavnik Petrovic. M235T polymorphism of the angiotensinogen gene and insertion/deletion polymorphism of the angiotensin-1 converting enzyme gene in essential arterial hypertension in Caucasians. Folia Biol. (Praha). 2007;53:69–70.
- Patnaik M, Pati P, Swain SN, et al. Association of angiotensin-converting enzyme and angiotensin-converting enzyme-2 gene polymorphisms with essential hypertension in the population of Odisha, India. Ann Hum Biol. 2014;41:143–150.
- Srivastava K, Sundriyal R, Meena PC, Bhatia J, Narang R, Saluja D. Association of angiotensin converting enzyme (insertion/deletion) gene polymorphism with essential hypertension in northern Indian subjects. Genet Test Mol Biomarkers. 2012;16:174–177.
