Abstract
Uranium is a radioactive heavy metal ubiquitous in the natural environment. In its chemical form, it is known to induce nephrotoxicity both in human and in animals. Its toxicity is dose and time dependent, also varies with form of uranium. In the present study, we assessed the nephrotoxicity induced by a single dose of uranyl nitrate (UN) in mice at different time intervals and recovery from its toxicity. Two doses of 2 and 4 mg/kg body weight of uranyl nitrate was injected intraperitoneally and animals were sacrificed after 1, 3, 5, 14, and 28 d of administration. Histopathological and biochemical alterations of post-UN dosing in comparison to control were evaluated. Tubular damage to about 75% was observed after 3 d (4 mg/kg) and the biochemical parameters such as serum creatinine, urea, and blood urea nitrogen levels were also significantly increased. Progression of tubular damage was not found after 5 d. Dose-dependent recovery of uranyl nitrate-treated animals was observed after 14 and 28 d of dosing. The concentration of uranium retained in kidney correlates with biochemical and histopathological analysis.
Introduction
Exposure to heavy metals is potentially harmful and is known to cause serious damage to different organs in humans and animals. Uranium (U) is a common heavy metal occurring in the environment with atomic number 92. Increased release of uranium to the environment is due to its wide spread application in civilian and military activities, nuclear power plant reactors, and counter weights in air craft.Citation1 The toxicity of uranium varies according to the chemical form, route of exposure, age, sex, body mass index, and species.Citation2 Solubility of uranium compounds also affects toxicity levels.Citation3
Animal studies indicate that majority of uranium (>95%) that enters the body is not absorbed and is excreted through feces.Citation4 Recovery from uranium assaults depends on the clearance of uranium from the body. Once absorbed, uranium forms soluble complexes with bicarbonate, citrate or proteins and is excreted through urine. Kidney and bone are the primary accumulators of absorbed uranium.Citation5 The biological half-life of uranium is 5–11 d in rat kidney, 14 d in rabbit kidney,Citation6 and 15 d in humans.
The spectrum of uranium-induced acute and long-term nephrotoxic effects has been extensively studied in animals and to some extent in humans.Citation7,Citation8 Regardless of the exposure, duration or route, the animal data provide strong evidence that kidney is the principal target organ for uranium induced toxicity with damage occurring principally to the proximal convoluted tubules.Citation9 It was demonstrated that uranium specially accumulates in the S3 segment of the proximal tubule, mild renal tubular lesions was observed at a dose 0.5 mg/kg by subcutaneous injection exposure of rat to uranium acetate.Citation10 It was reported that maximal effect on kidney is seen after 5 d of uranyl acetate oral administration with dietary consumption ad libitum oral administration in Swiss albino mice.Citation11 A single dose of 5 mg/kg of uranyl nitrate induced renal damage after 2 d in C57 BI/6J mice has been reported.Citation12 However, uranium-induced nephrotoxicity, tissue concentration, and recovery from the assault in mice are less studied with respect to time and dose and with this background, the present study was designed to evaluate the progressive kidney damage induced by single intraperitoneal dose of UN at different time intervals in mice with respect to biochemical and histopathological analyses and its recovery
Materials and methods
Animals and administration of uranyl nitrate
A total of 40 male Swiss albino mice of 8–10 weeks of age, weighing approximately 25 ± 5 g were selected for the study from an inbred colony maintained under the controlled conditions of 23 ± 2 °C, relative humidity of 55 ± 5%, and 12 h photoperiod with sterile food and water. Experiments were conducted after obtaining approval (No. 1/2014) by the Institutional animal ethics committee. The animal care and handling were according to the Institutional guidelines for animal experimentation. Animals were treated with a single intraperitoneal dose of 2 and 4 mg/kg of uranyl nitrate hexahydrate (MW = 502.13) dissolved in 0.9% saline. Control animals were treated with 0.9% saline. The animals were followed for a time period of 1, 3, 5, 14, and 28 d. Five animals were used in each experimental group. The body weight of the animal before and after dosing was recorded. Animals were sacrificed by euthanizing dose of sodium thiopentone after 1, 3, 5, 14, and 28 d of exposure, respectively, and changes in kidney size, weight, histopathology, biochemical parameters, and concentrations of uranium in kidney tissue were observed.
Biochemical parameters
Blood samples were collected from sacrificed animals by cardiac puncture and allowed to clot. The serum was separated after centrifugation at 1000 rpm for 10 min and used for the estimation of creatinine, urea and blood urea nitrogen (BUN) using diagnostic kits (Aggappe Diagnostic Ltd, Kochi, Kerala, India) in a biochemical analyzer (MispaPlus, Kerala, India) as per the protocols of the manufacturer.
Histopathological studies
Kidney tissues were fixed in 10% neutral-buffered formalin solution, dehydrated in graded alcohol, and then embedded in paraffin. For detection of tubular damage, the kidney sections were stained with hematoxylin and eosin. The degree of damage induced by uranyl nitrate was assessed semi quantitatively at 400× magnification using 20 randomly selected fields under a light microscope (Motic BA 210, Motic Incorporation Ltd., Hong Kong, Hong Kong).
Estimation of uranium content in kidney
Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) was used to determine the concentration of uranium in kidney. Freshly collected kidney tissue samples were weighed and digested using nitric acid followed by dilution with deionized water and used for uranium analysis by ICP-AES.
Statistical analysis
All data are expressed as mean ± standard error and significant difference between UN exposed and control group were analyzed using one-way analysis of variance (ANOVA) followed by Tukeys post hoc test (Megastat version 10.2) and the concentration of uranium retained and the percentage of tubular of tubular damage were statistically determined using Kruskal–Wallis test, p < 0.01 was considered as significant.
Results
Body weight and organ weight
Significant loss in body weight was observed in animals treated with 4 mg/kg after 3 and 5 d (p< 0.01). Body weight decrease of about 5% and 6% was observed after 3 and 5 d (4 mg/kg) of dosing, respectively, when compared with control. All UN-treated mice started to show weight loss from 3 d and normalization of body weight on 9 d onwards. No significant difference in mean kidney weights between control and exposed groups was evidenced.
Biochemical parameters
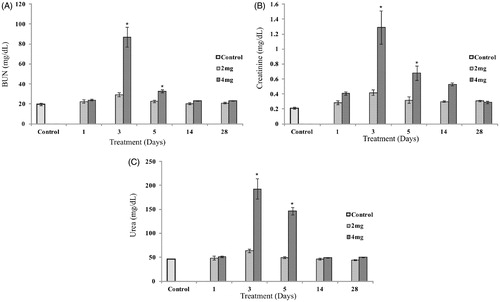
Biochemical parameters such as serum creatinine, urea and BUN in the UN-treated and control animals is presented in (). A significant increase in serum creatinine, urea, and BUN was observed in animals treated with 4 mg/kg after 3 and 5 d (p < 0.01) and then returned to normal after 14 d. No significant increase in serum parameters was evident in animals treated with 2 mg/kg after 1, 3, 5, 14, and 28 d compared to control.
Figure 1. Effect of UN on serum creatinine, BUN, and urea in animals compared with control. Data are represented as mean ± SE. Statistical significance was determined using one-way analysis of variance followed by Tukeys post hoc test (p < 0.01), * indicates significance (df = 4). Serum creatinine, BUN, and urea after 3 and 5 d of treatment are significantly different, compared with control and other treatment groups.
Concentration of uranium in kidney tissue
ICP-AES analysis indicated that the concentration of uranium retained in kidney tissue of animals treated with 4 mg/kg after 3 and 5 d were significantly higher than in animals after 1 and 14 d and 28 d of treatment (p < 0.01). Uranium concentration in kidney tissue of animals treated with 2 mg/kg after 1, 14, and 28 d of treatment were under the detection limit. Animals dosed with 2 mg/kg of UN retained less uranium in kidney compared to animals dosed with 4 mg/kg of UN after 3 and 5 d of treatment ()
Table 1. The amount of uranium retained in kidney tissue of Swiss albino mice treated with uranyl nitrate.
Histopathology of kidney tissue
Tubular damage was scored based on parameters such as cast formation in tubules, tubular dilation, degeneration of epithelial tissue, and damage to brush borders. After 1 d of dosing no damage was found in both 2 and 4 mg/kg of UN-treated animals (). Animals dosed with 2 mg/kg of UN after 3 d showed tubular damage (). Tubular damage was evident and most pronounced after 3 d in animals dosed with 4 mg/kg, both cortical and medullary regions of kidney showed critical damage. We found significant tubular damage in 4 mg/kg UN (p < 0.01) after 3 d compared with 2 mg/kg 3 d (). Tubular damage observed in animals exposed to 4 mg/kg of UN () was significant (p < 0.01) compared with 2 mg/kg UN after 5 d. Animals dosed with 2 mg/kg returned to normal by 14 d (). In animals exposed to 4 mg/kg after 14 d showed recovery with the clearance of cellular debris from the nephrons, however, complete normalization was not attained (). Almost complete recovery was observed in animals treated with 2 mg/kg after 28 d (). Delayed tubular regeneration or persistence of minor tubular damage was noticed in animals treated with 4 mg/kg UN after 28 d of dosing (). The percentage of tubular damage is presented in
Figure 2. Histopathology of kidney tissue of control and uranyl nitrate treatment groups. Cortical region of kidney: (A and B) (Control), (C and D) (1 d, 2 and 4 mg/kg) – intact tubular epithelium no damage, E (3 d, 2 mg/kg) – tubular damage, loss of microvilli, F (3 d,4 mg/kg) – extensive necrosis and cast formation in tubules, G (5 d, 2 mg/kg) – necrosis of proximal tubular epithelium, H (5 d, 4 mg/kg) – extensive necrosis of proximal tubular epithelium, I (14 d, 2 mg/kg) – tubular regeneration, J (14 d, 4 mg/kg) – basophilic regenerating epithelium lines most of the proximal tubules, K (28 d, 2 mg/kg) – intact tubular epithelium, L (28 d,4 mg/kg) – advanced regeneration of tubular epithelium.
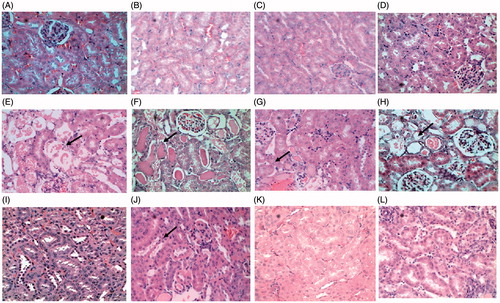
Table 2. Histopathological changes of kidney tissues of Swiss albino mice treated with uranyl nitrate.
Discussion
The toxic effects of uranium both in human and animals have been proved by several acute and chronic studies.Citation13,Citation14 It is well established that kidney is the principle target organ for heavy metal toxicity with damage primarily occurring in the proximal convoluted tubules.Citation9 Our aim is to determine progressive kidney damage induced by single intraperitoneal dose of uranyl nitrate with respect to histopathology and biochemical parameters at different time intervals. In the present study, we observed that a single dose of 2 mg/kg and 4 mg/kg of UN did not elicit any toxic responses after 1 d of dosing with respect to both histopathology and biochemical changes (p > 0.01). Biochemical parameters (creatinine, urea, BUN) were normal compared with control in animals treated with 2 and 4 mg/kg after 1 d and also no histopathological alternations such as tubular damage was observed. A similar study performed on rat showed that at low doses of 0.1, 0.3, and 1 mg/kg (single exposure) of uranyl acetate via intra muscular route did not show any significant increase in serum creatinine and BUN compared with control after 24 h.Citation15 In contrast, a study on male Wistar rats showed single injection of uranyl acetate at different doses of 0.5, 1, and2 mg/kg, i.p.(single exposure) caused a significant increase in serum blood nitrogen and creatinine levels after 1 d of dosing.Citation16 Single cell necrosis and mild degeneration were observed in rat treated with 1 mg/kg of UN after 1 d.Citation17. Such variations were observed in different studies in which the toxic responses may be due to the difference in route of administration and also species selected for the study. In our study, 1 d exposure to UN in mice may not be the exposure time to elicit a toxic response since no amount of uranium is retained in kidney or uranium retained is negligible to elicit toxicity.
Significant nephrotoxicity (p < 0.01) was observed in animals treated with 4 mg/kg of UN after 3 and 5 d with respect to biochemical parameters serum creatinine, urea, and BUN significantly increased compared with control. Similar results were also observed where in serum creatinine and BUN levels were significantly increased on 3 and 5 d after the administration of uranyl acetate to mice during a period of 5 d with dietary consumption ad libitum.Citation11 We observed histopathological changes indicating critical toxicity of about 75% tubular damage in animals after 3d (4 mg/kg) with increased loss of tubular epithelial lining, damage to brush border and cast formation within damaged tubules, epithelial cell degeneration, and granular deposits in tubular lumens with evidence of tubular epithelial cell desquamation and lymphocytic infiltration around proximal convoluted tubule. Animals treated with 4 mg/kg UN after 5 d showed 43% tubular damage. Earlier studies have reported histological alterations such as partial degeneration, necrosis and cast formation in proximal convoluted tubule although glomeruli remained intact.Citation18 A study on Sprague–Dawley male rat treated with uranyl acetate showed increased serum creatinine and tubular damage, which reached peak at day 5 but returned to base level by 14 d.Citation19 According to the result we obtained, nephrotoxic effects were found to be maximum at 3 d (4 mg/kg) and further progression of tubular damage was not noticed after 5 d. Our results also shows dose-dependent recovery, in animals dosed with 2 mg/kg after 14 and 28 d showed almost complete recovery with respect to both biochemical and histopathological parameters. Normalization of serum creatinine and urea levels due to increased clearance of urea and creatinine in urine and numerous regenerating cells began to reline the tubules and almost all the tubules regenerated in 2 mg/kg UN after 28 d. Regeneration of most of the damaged tubule with increased number of nuclei was evident in animals treated with 4 mg/kg, after 14 d and 28 d, most of the casts formed in the tubules was cleared and only about 6% of damage was persistent. It has been suggested that dedifferentiation of mature tubular cells plays a role in regeneration after acute kidney injury. Proximal tubular cells can regenerate through dedifferentiation of mature tubular cells, which can result in effective and rapid repair of focal proximal tubular lesions.Citation20
Recent clinical studies have reported the altered tubular function at uranium concentrations well below 3 μg uranium/gram concentration.Citation21 Recently, a chronic uranium exposure study reported that no nephrotoxic effect was found at kidney uranium concentration 6 μg/g of kidney.Citation22 We observed that the concentration of uranium retained in kidney tissue after administration of UN varies depending on the dose of UN administrated. Animals dosed with, 4 mg/kg of UN after 3 and 5 d retained high levels of uranium in kidney compared with 2 mg/kg of UN and no detectable amount of uranium was found after 14 and 28 d in 2 mg/kg of UN-treated animals. Animals treated with 4 mg/kg UN after 14 d retained uranium in kidney, this correlates with the histopathological profile that is persistence of 12% damage after 14 d. The concentration of uranium retained in kidney was well correlated with the biochemical and histopathological profiles. We observed the amount of uranium retained after 1d 4 mg/kg can be considered as concentration which may not induce nephrotoxicity to alter tubular function (5 μg/g of kidney). Bioaccumulation of uranium depends on the dose and the exposure time.
The study concludes that no observable nephrotoxicity was found in animals treated with a single dose of 2 mg and 4 mg/kg of UN nitrate after 1d of post-dosing. Single dose of 4 mg/kg of UN after 3 d showed tubular damage of about 75% in mice and further progression of damage was not observed after 5 d. Study also reveals that recovery after UN toxicity found to be dose dependent. The concentration of uranium retained in kidney is well correlated with dose of UN exposed. The result obtained can be further explored to understand the mechanism through which kidney retains its homeostasis after UN assault.
Acknowledgements
The authors acknowledge IIT Bombay for extending the ICP-AES analysis facility.
Disclosure statement
The authors declare that there is no conflict among authors.
Funding information
The authors acknowledge BRNS (Project no. 2010/36/69-BRNS) for the financial support. The author V. P. Sangeetha acknowledges Yenepoya University for the junior research fellowship.
References
- Priyamvada S, Khan SA, Khan MW, et al. Studies on the protective effect of dietary fish oil on uranyl-nitrate-induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot Essent Fatty Acids. 2009;82:35–44.
- Langmuir D. Aqueous Environmental Geochemistry. New Jersey: Prentice Hall; 1997:486–547.
- ATSDR. Toxicological Profile for Uranium. Atlanta, GA: Agency for Toxic Substances & Disease Registry; 2013.
- Brugge D, Buchner V. Health effects of uranium: New research findings. Rev Environ Health. 2011;26:231–249.
- Katz SA. The chemistry and toxicology of depleted uranium. Toxics. 2014;2:50–78.
- Tracy BL, Quinn JM, Lahey J, et al. Absorption and retention of uranium from drinking water by rats and rabbits. Health Phys. 1992;62:65–73.
- Vicente-Vicente L, Quiros Y, Pérez-Barriocanal F, et al. Nephrotoxicity of uranium: Pathophysiological, diagnostic and therapeutic perspectives. Toxicol Sci. 2010;118:324–347.
- Gilman AP, Villeuve DC, Secours VE, et al. Uranyl nitrate: 28-day and 91-day toxicity studies in the Sprague–Dawley rat. Toxicol Sci. 1998;41:117–128.
- Kathren RL, Burklin RK. Acute chemical toxicity of uranium. Health Phys. 2008;94:170–179.
- Homma‐Takeda S, Kokubo T, Terada Y, et al. Uranium dynamics and developmental sensitivity in rat kidney. J Appl Toxicol. 2013;33:685–694.
- Ozmen M, Yurekli M. Subacute toxicity of uranyl acetate in Swiss-Albino mice. Environ Toxicol Pharmacol. 1998;6:111–115.
- Taulan M, Paquet F, Argiles A, Demaille J, Romey MC. Comprehensive analysis of the renal transcriptional response to acuteuranyl nitrate exposure. BMC Genomics. 2006;7:2–317.
- Kurttio P, Harmoinen A, Saha H, et al. Kidney toxicity of ingested uranium from drinking water. Am J Kidney Dis. 2006;47:972–982.
- Taulan M, Paquet F, Maubert C, Delissen O. Renal toxicogenomic response to chronicuranyl nitrate insult in mice. Environ Health Perspect. 2004;112:1628–1635.
- Zimmerman KL, Barber DS, Ehrich MF, et al. Temporal clinical chemistry and microscopic renal effects following acute uranyl acetate exposure. J Toxicol Pathol. 2007;35:1000–1009.
- Shaki F, Hosseini MJ, Ghazi KM, Pourahmad J. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim Biophys Acta. 2012;1820:1940–1950.
- Ohmachi Y, Imamura T, Ikeda M, et al. Sodium bicarbonate protects uranium-induced acute nephrotoxicity through uranium-decorporation by urinary alkalinization in rats. J Toxicol Pathol. 2014;28:65–71.
- Protective role of Ginkgo biloba against hepatotoxicity and nephrotoxicity in uranium-treated mice. J Med Food. 2010;13:179–188.
- Sano K, Fujigaki Y, Miyaji T, et al. Role of apoptosis in uranyl acetate-induced acute renal failure and acquired resistance to uranyl acetate. Kidney Int. 2000;57:1560–1570.
- Fujigaki Y. Different modes of renal proximal tubule regeneration in health and disease. World J Nephrol. 2012;1:92–199.
- Squibb KS, Leggett RW, McDiarmid MA. Prediction of renal concentrations of depleted uranium and radiation dose in Gulf War veterans with embedded shrapnel. Health Phys. 2005;89:267–273.
- Poisson C, Stefani J, Manens L, et al. Chronic uranium exposure dose-dependently induces glutathione in rats without any nephrotoxicity. Free Radic Res. 2014;48:1218–1231.
