Abstract
To investigate the role of mast cells (MCs) renal infiltration in the progression of human anti-GBM nephritis, 38 patients diagnosed with anti-GBM nephritis were enrolled. Renal biopsies were performed. Immunohistochemistry was conducted to detect MCs in renal tissues. Patients were divided into group 1 (MCs <50 mm−2, n = 18) and group 2 (MCs ≥50 mm−2, n = 20) according to the infiltrating renal MC count. The clinical–pathological indices were compared. And, correlation between MCs and the clinical–pathological indices was analyzed. Patients of group 2 had more severe renal dysfunctions, expressed as higher levels of serum creatinine (SCr 8.95 ± 3.66 vs. 4.75 ± 2.73 mg/dL, p < 0.001), urine retinol-binding protein (RBP 29.8 ± 13.9 vs. 15.7 ± 11.5 mg/dL, p = 0.005), and lower urinary osmotic pressure. Pathologically, patients of group 2 had a higher percentage of fibrous/fibrocellular crescents (66.7 ± 21.9 vs. 47.0 ± 33.6%, p = 0.037) but a lower percentage of cellular crescents. More CD8 (268 mm−2 vs. 180 mm−2, p = 0.045) and CD68 (268 mm−2 vs. 180 mm−2, p = 0.045) positive cells infiltrating the interstitium were observed in group 2. Furthermore, renal MCs correlated significantly with the total number of crescents and the tubular interstitial CD8 and CD68 positive cells. And, the number of MCs was associated with the histological types. The renal function was significantly different between the two groups at presentation. However, at 3 and 6 month follow-up, the patient outcome was associated with the histological types. Our study showed that MC infiltrations were associated with chronic lesions in anti-GBM nephritis and may be involved in the loss of renal function with pathological changes.
Introduction
Mast cells (MCs) are derived from hematopoietic progenitor cells and migrate through vascularized tissue to complete their maturation.Citation1 They participate in many steps of the inflammatory response, either through systemic activation or local secretion of inflammatory mediators. In past decades, MCs have been demonstrated to play an important role in allergic inflammatory conditions, such as asthma, and to increase in number in chronic inflammatory conditions.Citation2 Their presence in tissue is often accompanied by fibrosis, such as liver cirrhosis, pulmonary fibrosis, and wound healing, suggesting that they might be involved in these pathologic conditions.Citation3 In recent years, increased number of MCs have been detected in various glomerular diseases, including most of the primary and secondary forms of glomerulonephritis, and in allograft rejection, as well as amyloidosis, renovascular ischemia, reflux nephropathy, polycystic kidney disease, and drug-induced nephropathy, primarily in experimental animal models.Citation4–Citation11 Recently, we reported that mast cells could promote renal inflammation and fibrosis and thus contribute to diabetic nephropathy through the release of bioactive substances, such as tryptase, by degranulation into the tubular interstitium.Citation12
Macrophages/monocytes are thought to play a central role in crescentic and tubulointerstitial lesions.Citation5,Citation13,Citation14 MCs are bone marrow-derived hematopoietic cells that share phenotypic characteristics with monocytes/macrophages. While monocyte infiltration into the glomeruli and interstitium has been intensively investigated and found to be the initial event in progressive glomerular crescent formation and interstitial alterations in most models of renal diseases,Citation6 MCs may also be implicated in the pathogenesis of crescentic glomerulonephritis (CGN).Citation7 Anti-GBM nephritis is a common CGN. Some studies have revealed that MCs may contribute to tubular interstitial fibrosis by synthesizing and releasing enzymes and chemokines. The role of MCs in anti-GBM nephritis has been demonstrated in the animal models. However, only a few patients have been examined, and evidence of the involvement of MCs in human anti-GBM nephritis is still scarce. In this study, the immunohistochemical localization of MCs in the kidney was examined in patients with anti-GBM nephritis, and its correlation with clinical and histopathological parameters was investigated.
Methods
Patients
Thirty-eight patients diagnosed with anti-GBM nephritis in our institute between 2001 and 2010 were enrolled in this study. Signed informed consent forms were obtained from all patients, and this study was approved by the Ethics Committee of Jinling Hospital. All patients underwent methylprednisolone pulse therapy followed by oral prednisone or mycophenolate mofetil or intravenous cyclophosphamide pulse therapy. Renal biopsies were performed in all patients. Clinical and pathological data were collected. There were few MCs infiltrating the glomeruli and peri-glomerular regions. Based on the infiltrating renal MC count, the patients were divided into group 1 (MCs <50 mm−2, n = 18) and group 2 (MCs ≥50 mm−2, n = 20). The clinical, laboratory, and pathological indices were compared between the two groups.
Clinical data, including the patients’ general condition, gender, age, course of disease, clinical manifestations and laboratory tests, including urinary protein, urinary sediment red blood cell count (RBC), the tubular function indicators [NAG (urinary N-acetyl-β-d-glucosaminidase) enzyme, RBP (urinary retinol-binding protein)], hemoglobin, serum creatinine (SCr) values and serological tests, such as anti-GBM antibody titers, complement component 3 (C3) and complement component 4 (C4), were recorded. Hypertension was defined as a resting adult systolic blood pressure ≥140 mmHg and (or) diastolic blood pressure ≥90 mmHg and pediatric systolic blood pressure ≥120 mmHg and (or) diastolic blood pressure ≥80 mmHg, and at least two repeated measures were performed for each patient. Microscopic hematuria was defined as a urinary sediment RBC ≥100,000/mL, and proteinuria as urinary protein excretion >0.4 g/24 h. Nephrotic syndrome was defined as urinary protein ≥3.5 g/24 h, and serum albumin < 30 g/L. Estimated glomerular filtration rate (eGFR) was calculated with the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation. Chronic kidney disease (CKD) was defined as eGFR <60 mL/min/1.73 m2 or markers of kidney damage for more than 3 months. End-stage of renal disease (ESRD) was defined as eGFR <15 mL/min/1.73 m2, initiation of dialysis or transplantation that continued for 3 months, or transplantation. And, chronic renal failure (CRF) was defined as SCr > 1.24 mg/dL for at least 3 months.
Histological examination
For light microscopy, renal biopsy specimens were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned at 2 μm thickness. Hematoxylin–eosin, periodic acid-Schiff’s reagent, Masson’s trichrome, and periodic acid methenamine silver staining were performed. Glomerular, tubular interstitial and vascular lesions in biopsies were recorded. Interstitial and tubular lesions were scored semi-quantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: interstitial fibrosis/tubular atrophy (0, 0%; 1, 0–25%; 2, 25–50%; 3, >50%). Routine paraffin sections were immunostained using a four PAP method to determine CD4, CD8, CD68 and proliferating cell nuclear antigen positive cells in the interstitium and glomeruli. If >90% of a crescent consists of extracellular matrix, then the term fibrous crescent is used. As long as the crescent contains cellular elements >90%, it is regarded as a cellular crescent. Biopsies were scored for glomerular lesions in the following order referring to the histopathologic classification of ANCA-associated glomerulonephritisCitation15: global sclerotic glomeruli, normal glomeruli, and cellular crescentic glomeruli. The biopsies in the focal category have 50% of the normal glomeruli that are not affected by the disease process. The crescentic category has biopsies with 50% of glomeruli with cellular crescents. Biopsies from the sclerotic category have 50% of glomeruli with global sclerosis. Any biopsies that do not fit into one of the categories on the basis of a predominant glomerular phenotype will automatically be included in the mixed category. For each renal biopsy, two independent observers assessed all the glomerular sections for all the immunohistochemical markers.
Detection of renal MCs
Immunohistochemical staining for tryptase and CD117 was carried out to enumerate the renal MCs. Briefly, formalin-fixed sections were deparaffinized with xylene and rehydrated with graded ethanols. The sections were autoclaved for antigen retrieval. Endogenous peroxidase was blocked with hydrogen peroxide. To reduce the background signal, the sections were blocked for 30 min in 10% fetal calf serum. We used anti-tryptase antibody and anti-CD117 antibody to distinguish MCs from other cell types. The samples were rinsed in PBS and incubated overnight at 4 °C with different first antibodies, such as mouse anti-human MC tryptase monoclonal antibody (Chemicon, Temecula, CA; 1:2000), rabbit anti-human CD117 (DAKO, Carpinteria, CA; 1:100) and mouse anti-human CD68 monoclonal antibody (DAKO; 1:100). After washing with PBS, the tissues were incubated with Envision reagent (DAKO) for 45 min, and the sections were washed and developed with diaminobenzidine. Each section was counterstained with hematoxylin. Normal homologous serum was used to replace the first antibody as a negative control. Renal sections stained against tryptase and CD117 were examined under a microscope at a high power field by two different researchers using a single blind approach. At least 20 randomly selected fields were examined for each section. The number of tryptase-positive cells (total MCs) in each section was counted by two independent researchers using a single blind approach. CD117 staining was only conducted on five sections of group 1 and five sections in group 2. And the numbers of CD117 positive cells also were counted (data not shown) ().
Figure 1. The distribution of MCs in patients with anti-GBM nephritis: immunohistochemical (IH) staining showing tryptase expression in the kidney of normal control (A), patients with anti-GBM nephritis in group 1 (B) and in group 2 (C) (IH, 200×).
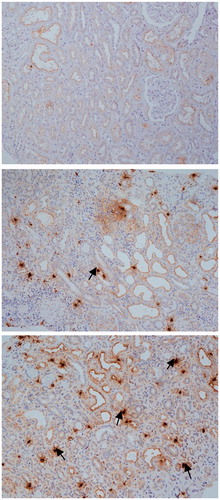
Figure 2. The distribution of MCs in patients with anti-GBM nephritis: immunohistochemical (IH) staining showing CD117 expression in the kidney of patients with anti-GBM nephritis in group 1 (A) and in group 2 (B) (IH, 200×).
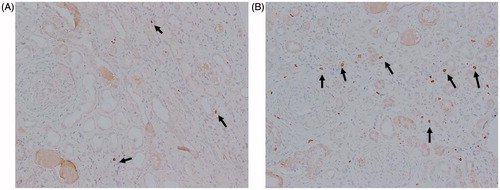
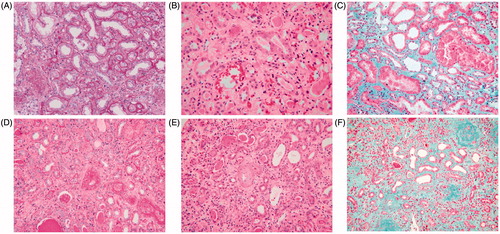
Figure 3. Renal pathology for hematoxylin–eosin, periodic acid-Schiff’s reagent, periodic acid methenamine, and Masson’s trichrome staining of patients in group 1 (A–C) and group 2 (D–F) (200×): it could be seen from the contrasts of pathology of the two groups that the patients in group 2 had more chronic lesion such as more serious degree of fibrosis and more inflammatory cell infiltration.
Statistics
All date were analyzed by using SPSS program 16.0 (SPSS, Inc., Chicago, IL) for Windows. Results were expressed as means ± standard deviation or median according to whether the data were normally distributed. Mean comparisons between two groups were examined for significant differences using the t-test and rate between the two groups were compared by using the Mann–Whitney U test or χ2 test, and Spearman correlation analyses were used to analyze the relationship among different parameters, with p values <0.05 indicating statistical significance.
Results
General conditions and clinical features
All patients showed rapidly progressive glomerulonephritis. More than half (65.8%) of the cases were accompanied by gross hematuria. Most of the patients (84.2%) had hypertension. The patients in group 2 (who were older in age) had a tendency of longer course of disease and higher percentage of gross hematuria and oliguria/anuria. More patients with CRF were distributed in group 2. However, there was no significant difference between the two groups ().
Table 1. General conditions and clinical features between two groups.
Laboratory findings
The patients in group 2 had higher levels of SCr (8.95 ± 3.66 vs. 4.75 ± 2.73 mg/dL, p < 0.001) and urine retinol-binding protein (RBP) (29.8 ± 13.9 vs. 15.7 ± 11.5 mg/L, p = 0.005) but lower urinary osmotic pressure. Although no significant differences were found between the two groups, the patients in group 2 had a lower degree of proteinuria, microscopic hematuria, and hypoproteinemia, and a more severe degree of anemia and a higher titer of anti-GBM antibodies ().
Table 2. Laboratory data between two groups.
Histological examination
The patients in group 2 had a higher percentage of glomerular sclerosis and Bowman’s wall rupture and more severe degree of fibrosis although no significant differences were observed between the two groups. These patients also had a higher percentage of fibrous/fibrocellular crescents (66.7 ± 21.9 vs. 47.0 ± 33.6%, p = 0.037), but a lower percentage of cellular crescents. There were more inflammatory cells, including CD4, CD8 and CD68 positive cells infiltrating the interstitium of group 2. Among these inflammatory cells, the infiltration of CD8 positive T cells (268 vs. 180 mm−2, p = 0.035) and CD68 positive cells (792 vs. 534 mm−2, p = 0.017) in the interstitium were significantly different between the two groups ( and ).
Table 3. Data of histopathology between two groups.
Table 4. The histopathologic types between two groups.
The crescentic type was more common in patients of group 1 (9/18, 50%) compared with group 2 (3/20, 15%). However, the number of MCs in the sclerotic type was higher than the other two types, especially compared with the crescentic type (p < 0.05). The detailed data are shown in .
Table 5. The number of MC in different type of histopathology.
Correlation analysis
The correlation analysis of renal MC infiltration with the clinical and pathological parameters is summarized in . The infiltration of renal MCs correlated significantly with RBP (r = 0.549, p = 0.001), albumin (r = 0.478, p = 0.002), SCr (r = 0.494, p= 0.002), fibrosis (r =0.393, p= 0.015), Bowman’s wall rupture (r = 0.388, p = 0.016), CD8 in the interstitium (r = 0.337, p = 0.041) and CD68 in the interstitium (r = 0.356, p = 0.033).
Table 6. Correlations between MCs with clinic pathological characteristics.
Follow-up and outcome
Four (10.5%) patient deaths occurred at presentation or during follow-up, and five patients were lost during follow-up. A total of 22/29 (75.9%) patients progressed to ESRD, with the median time for renal survival of 9 weeks (95% CI, 5–15weeks). Of these, 7/29 (24.1%) patients progressed to CRF without dialysis. No significant differences in the rate of progression to ESRD or CRF were observed between the two groups. The decrease of eGFR in group 1 was slower than that in group 2, but this decrease was not significantly different except at presentation. The decrease of eGFR in patients with crescentic lesions was significantly slower than that in patients with sclerotic lesions. The decreases of eGFR for different patterns of histology and the degrees of renal MC infiltration are shown in and .
Table 7. The eGFR changes among the histopathologic types and between two groups.
Discussion
As an important member of inflammatory cells, MCs are often present in a large number of infiltrations in areas of inflammation and fibrosis. Thus far, reports about the role of MCs in kidney diseases have described the existence of MCs in the interstitial area of human renal biopsy tissues from patients with various renal diseases, including almost all primary and secondary glomerulonephritis. The role of MCs in the pathogenesis of crescent glomerulonephritis has been expanding. However, despite a few studies in experimental animals with inconsistent results,Citation8–10 the role of MCs in human anti-GBM nephritis remained unclear.
In this study, we demonstrated that there was a significant interstitial accumulation of MCs in human anti-GBM nephritis. The patients with severe renal MC infiltration (group 2) were older and had a longer course of disease, higher percentages of gross hematuria, oliguria/anuria, a more severe degree of anemia and less proteinuria and microscopic hematuria, as well as a higher level of anti-GBM antibodies. Moreover, these patients had more serious renal dysfunctions, which were expressed as higher levels of SCr and urine RBP, but lower urinary osmotic pressure. Additionally, these patients had a higher percentage of glomerular sclerosis, Bowman’s wall rupture and more severe degree of fibrosis, as well as a higher percentage of fibrous/fibrocellular crescents but a lower percentage of cellular crescents. There were more inflammatory cells infiltrated in the interstitium of group 2. In addition, renal MCs correlated significantly with the total number of crescents and the tubular interstitial inflammatory cells, including CD68 positive cells and CD8 positive T lymphocytes. All the above results possibly reflected chronic lesions in anti-GBM nephritis and demonstrated that MCs might be involved in renal chronic damage.
It has been reported that in patients with clinical features, such as high SCr, oliguria or anuria, a late diagnosis have led to a poor renal outcome,Citation11,Citation16–18 and SCr at onset was an independent predictor of ESRD. Regarding our data of the patients’ general conditions and clinical features, it could be discerned that although there was no significant difference, the group with more MC infiltration showed a tendency for an older age, a longer course of disease, a lower percentage of AKI and a higher percentage of CRF, which may be associated with chronic kidney lesions. The clinical data showed that renal dysfunctions in these patients were more severe, which were expressed as higher levels of SCr and urine RBP but lower urinary osmotic pressure, higher percentages of oliguria/anuria, a more severe degree of anemia and less proteinuria and microscopic hematuria, as well as a higher level of anti-GBM antibodies and more infiltration of MCs that might be related to chronic renal lesions. In these patients, the increased presence of MC infiltration indirectly demonstrated that MC infiltration was associated with chronic lesion.
In this study, all patients had more than 40% of crescents, with an average of 60.3 ± 33.7%, and more than one-third of patients had more than 85% of crescents. The patients had an average of 57.3% of fibrosis/fibrocellular crescents, which were present through the late diagnosis in our patients. It has been recognized that with the progression of the disease process, histologic examination usually showed a fibrocellular pattern followed by a fibrous transformation of the glomerular crescents, together with tubulointerstitial damage, including interstitial fibrosis. Comparing the condition between the two groups, fibrosis/fibrocellular crescents and globally sclerotic glomeruli accounted for a greater proportion in group 2. The more fibrotic/fibrocellular crescents and globally sclerotic glomeruli further confirmed that more MC infiltration was associated with the late process of crescent formation, which was difficult to reverse by immunosuppressive therapy and always lead to ESRD. Comparing three types of crescents, the number of MCs in the sclerotic type was higher than that in the other two types. A study by Tóth showed a significantly higher density of MCs in forms of GN associated with fibrocellular crescents.Citation7 Timoshanko et al. suggested that MCs could aggravate CGN.Citation8 Moreover, together with MC-mediated infiltration with T cells, macrophages are reported in the study of Hochegger et al.Citation9 It was recognized that most forms of human crescentic GN are associated with Th1 predominant nephritogenic immunity and the accumulation of glomerular T cell effectors.Citation19 Accordingly, MCs may mediate and enhance the development of crescent formation. However, further studies are needed to clarify these pathogenic mechanisms.
The progression of CRF is characterized by glomerulosclerosis and tubulointerstitial fibrosis.Citation20 In addition to the glomerular damages, our data also showed that the accumulation of MCs in the interstitium correlated closely with tubulointerstitial damage. In this study, the patients with more MC infiltration had more tubular lesions, which was consistent with the clinical characteristics (higher levels of SCr and RBP), indicating that the tubular injury may be another factor responsible for the renal damage. Furthermore, the extent of MC infiltration tended to be significantly higher in patients undergoing hemodialysis. Moreover, the involvement of MCs in tubular interstitial injury was also supported by the results of the correlation analysis, which showed that MCs significantly correlated with the levels of RBP, SCr and the degree of fibrosis. Based on these observations, our hypothesis would be that MCs could contribute to renal function loss through promotion of tubular interstitial injury. Similar results with interstitial lesions were also reported for other renal diseases.Citation4,Citation21 Additionally, the intensity and extent of tubulointerstitial damage was reported to be one of the strongest determinants of progressive functional decline.Citation22 These observations indicated that MCs could not be ignored in tubulointerstitial damage. Some experimental studies have revealed possible mechanisms through which MCs could promote the tubulointerstitial damage. The potential significance of the close association among interstitial fibrosis, MC accumulation, and the role of the local renal renin–angiotensin system (RAS) are highlighted in recent studies,Citation23–26 showing that MCs induce RAS activation. As MCs are capable of releasing various fibrogenic factors and growth factors, including histamine, tryptase, transforming growth factor, and basic fibroblast growth factor, they possess mitogenic activity for fibroblasts and enhanced collagen synthesis.Citation27
Correlation analysis also demonstrated that more MC infiltration was associated with a higher percentage of sclerotic glomeruli and was more severe with the degree of renal interstitial fibrosis. The renal function loss caused by MCs might be associated with pathological changes of both glomerular and tubular components, which indicates that MCs may be associated with the late phase of the disease, further demonstrating our conclusion that MCs are implicated in chronic lesions.
There was more infiltration with CD8 cells, CD4 cells and CD68 cells in interstitium of group 2 (especially CD8 cells and CD68 cells, which showed a significant difference between the two groups and a positive correction with the accumulation of MCs). As mentioned above, MCs could mediate and enhance the infiltration of T cells and macrophages. Macrophages have been shown to have an important role in crescent formation. The macrophage accumulation correlated with the degree of renal dysfunction and was predictive of disease progression.Citation28 In view of the positive correlations between MCs and CD68 positive cells (which mainly present macrophages), we could hypothesize that MCs enhanced the progression of the disease partially through their interaction with macrophages. It has been reported that MCs not only serve as effector cells but also play a role in inducing T cell activation, recruitment, proliferation, and cytokine secretion in inflammation.Citation29 T cells may induce the inflammation in the interstitium and aggravate the renal injury. That T cell–MC interaction supported the development of T cell responses is the likely explanation for the injurious role of MCs. These hypotheses were also supported by other studies that revealed that the interaction of MCs and other immune cells could promote the exacerbation of disease. MCs produce and release several chemical mediators. Some of these mediators can modulate biological responses by regulating cytokine expression or inflammatory cell infiltration.Citation30 Timoshanko et al. also demonstrated a role for MCs in inducing functional renal injury through the mechanism of infiltrating MCs that recruit delayed-type hypersensitivity (DTH) effector leukocytes. These local effects were consistent with studies showing a role for MCs in dermal DTH.Citation31 The correlation between MCs and some inflammatory cells (CD8 T cells and CD68 cells) may suggest that MCs play a role in promoting renal inflammation in anti-GBM nephritis.
In conclusion, some investigations have demonstrated that MCs may play an adverse role in the chronic inflammation, angiogenesis, tissue remodeling and development of tubulointerstitial fibrosis in various types of renal disease, such as diabetic nephropathy, IgA nephropathy and AKI. Our study shows that MC infiltration was associated with chronic lesions in anti-GBM nephritis and may be implicated in the loss of renal function with pathological changes due to both glomerular and tubular-interstitial damage and inflammation. Because of the poor prognosis of anti-GBM nephritis, the studies of MCs in this disease might provide a new approach to minimize the loss of kidney function.
Disclosure statement
The authors have no financial or other conflicts of interest to disclose. This work was supported by Jiangsu Province basic research project of Natural Science Foundation (BK20131327).
References
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76.
- Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y. Mast cells and inflammatory kidney disease. Immunol Rev. 2007;217:79–95.
- Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol. 1997;26:1042–1054.
- Roberts IS, Brenchley PE. Mast cells: The forgotten cells of renal fibrosis. J Clin Pathol. 2000;53:858–862.
- Holdsworth SR, Allen DE, Thomson NM, Glasgow EF, Atkins RC. Histochemistry of glomerular cells in animal models of crescentic glomerulonephritis. Pathology. 1980;12:339–346.
- Zheng JM, Yao GH, Cheng Z, et al. Pathogenic role of mast cells in the development of diabetic nephropathy: A study of patients at different stages of the disease. Diabetologia. 2012;55:801–811.
- Tóth T, Tóth-Jakatics R, Jimi S, Ihara M, Urata H, Takebayashi S. Mast cells in rapidly progressive glomerulonephritis. J Am Soc Nephrol. 1999;10:1498–1505.
- Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:150–159.
- Hochegger K, Siebenhaar F, Vielhauer V, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35:3074–3082.
- Kanamaru Y, Scandiuzzi L, Essig M, et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J Immunol. 2006;176:5607–5615.
- Cui Z, Zhao MH, Xin G, Wang HY. Characteristics and prognosis of Chinese patients with antiglomerular basement membrane disease. Nephron Clin Pract. 2005;99:c49–c55.
- Kondo S, Kagami S, Kido H, Strutz F, Müller GA, Kuroda Y. Role of mast cell tryptase in renal interstitial fibrosis. J Am Soc Nephrol. 2001;12:1668–1676.
- Ferrario F, Castiglione A, Colasanti G, Barbiano di Belgioioso G, Bertoli S, D’Amico G. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28:513–519.
- Tang Z, Wu Y, Hu W, et al. The distribution and significance of renal infiltrating cells in patients with diffuse crescentic glomerulonephritis. Chin Med J (Engl). 2001;114:1267–1269.
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636.
- Daly C, Conlon PJ, Medwar W, Walshe JJ. Characteristics and outcome of anti-glomerular basement membrane disease: A single-center experience. Ren Fail. 1996;18:105–112.
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042.
- Jindal KK. Management of idiopathic crescentic and diffuse proliferative glomerulonephritis: Evidence-based recommendations. Kidney Int Suppl. 1999;70:S33–S40.
- Kitching AR, Holdsworth SR, Tipping PG. Crescentic glomerulonephritis – A manifestation of a nephritogenic Th1 response? Histol Histopathol. 2000;15:993–1003.
- El Nahas AM. Glomerulosclerosis: Intrinsic and extrinsic pathways. Nephrol Dial Transplant. 1996;11:773–777.
- Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol. 2008;19:2254–2261.
- El-Koraie AF, Baddour NM, Adam AG, El Kashef EH, El Nahas AM. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int. 2001;60:167–172.
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin–angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287.
- Mackins CJ, Kano S, Seyedi N, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070.
- Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int. 2003;64:906–913.
- Silver RB, Reid AC, Mackins CJ, et al. Mast cells: A unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–13612.
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152.
- Atkins RC. Macrophages in renal injury. Am J Kidney Dis. 1998;31:xlv–xlvii.
- Bulfone-Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol. 2015;6:394.
- Villa I, Skokos D, Tkaczyk C, et al. Capacity of mouse mast cells to prime T cells and to induce specific antibody responses in vivo. Immunology. 2001;102:165–172.
- Tanda S, Mori Y, Kimura T, et al. Histamine ameliorates anti-glomerular basement membrane antibody-induced glomerulonephritis in rats. Kidney Int. 2007;72:608–613.
