Abstract
This study investigated whether oxymatrine (OMT) treatment can ameliorate renal interstitial fibrosis in unilateral ureteral obstruction (UUO) mice model. Moreover, the potential mechanisms of such treatment were analyzed. Twenty-four C57/BL6 mice were randomly divided into three groups, namely sham group, vehicle plus unilateral ureteral obstruction (UUO)-treated group, and 100 mg/kg/d OMT plus UUO-treated group. All mice were euthanized seven days after surgery, and their kidneys were harvested. Renal injury, fibrosis, expression of proinflammatory cytokines, and the transforming growth factor-β1/Smads (TGF-β/Smads) and nuclear factor-kappa B (NF-κB)-signaling pathways were assessed. The results showed OMT significantly prevented kidney injury and fibrosis, as evidenced by decreased expression of collagen-1 and fibronectin. Furthermore, OMT administration inhibited the release of inflammatory factors including tumor necrosis factor-α, (TNF-α) interleukin-1β (IL-1β), and interleukin-6 (IL-6), as well as phosphorylated NF-κB p65. In addition, OMT blocked the activation of myofibroblasts by inhibiting the TGF-β/Smad3-signaling pathway. The findings indicate that OMT-attenuated renal fibrosis and inflammation, and this renoprotective effect may be ascribed to the inactivation of the TGF-β/Smad3 and NF-κB p65 pathways.
Introduction
Chronic kidney disease (CKD) is a worldwide health problem affecting approximately 10% of the population in developed countries.Citation1 Most CKDs, which share common pathological features such as tubulointerstitial fibrosis, can progress into end-stage renal disease (ESRD).Citation2 Renal fibrosis is characterized by excessive production and disposition of extracellular matrix (ECM), renal epithelial cell injury, interstitial inflammation, and activation of myofibroblasts, ultimately resulting in fibrotic lesions and tissue scarring.Citation3 During the obstruction process in the kidney, resident interstitial fibroblasts differentiate into myofibroblasts, thereby actively synthesizing excessive ECM components and causing renal fibrosis.Citation4 Inflammatory cells infiltrate into the renal interstitium and release plenty of cytokines and chemokines to sustain the inflammatory response contributed to fibrogenesis.Citation5 Among these cytokines, TGF-β1 is thought to be the central mediator initiating renal fibrosis. During fibrogenesis, the release of TGF-β1 activates TGF-β type-I receptor (TβRI), leading to the phosphorylation of Smad2/3, followed by translocation of activated R-Smads into the nucleus, thus increasing the transcription of ECM genes to increase the production of matrix.Citation6 As a result, targeting the TGF-β/Smads-signaling pathway may represent a specific and effective therapy for CKD associated with renal fibrosis.Citation7
Oxymatrine (OMT) is a natural component extracted from the roots of traditional Chinese herb named Radix Sophorae flavescentis (Kushen), which has been widely reported to present various pharmacological effects, such as antitumor,Citation8 anti-inflammation,Citation9 cardioprotection,Citation10 and antifibrosis.Citation11 Recently, evidence indicated that OMT exerted prominent protective effects on hepaticCitation12 and myocardialCitation13 fibrosis, as well as an increasing protective effect against kidney injuryCitation14; however, few studies have focused on the role of OMT in kidney fibrosis. Therefore, the current research investigated whether OMT can attenuate renal fibrosis in a unilateral ureteral obstruction (UUO) model, as well as analyzed the potential mechanisms of such treatment.
Methods and materials
Animal model
A total of 24 eight-week-old C57BL/6 mice were purchased from Wuhan University Center for Animal Experiment (Wuhan, China) and housed in Renmin Hospital of Wuhan University Animal Center. Animal studies were conducted with an approved protocol by the Institutional Animal Care and Use Committee of the University of Wuhan, China. UUO method was performed using an established procedure as previously described.Citation15 The mice were randomized into three groups (n = 8 each group): sham operation, UUO plus vehicle (UUO + V) and UUO plus 100 mg/kg/d body weight OMT (Nanjing Spring and Autumn Biological Engineering Co., Ltd., Nanjing, China). The dosage of OMT was selected on the basis of our pilot test results to ensure that pathology staining of the UUO model was improved. OMT was dissolved in 0.9% sodium chloride, and the mice received intraperitoneal injection of 100 mg/kg/d OMT daily for six days after surgery. The vehicle treatment was administered with the same volume of 0.9% sodium chloride. At seven days after surgery, the mice were euthanized, and kidneys were collected.
Western blot analysis
Tissues lysates were extracted as indicated and homogenized in a sodium dodecyl sulfate (SDS) buffer with 1% cocktail (Biyuntian, Haimen, China) for 30 min on ice. Afterward, samples were centrifuged at 13,000 ×g for 20 min and then heated at 95 °C and separated by SDS-polyacrylamide gel electrophoresis. Subsequently, the samples were electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA) and then blocked and incubated with primary antibodies, namely, anti-TGF-β1, p-smad2, smad2, p-smad3, smad3, (Cell Signaling Technology, Danvers, MA), -SMA, vimentin, and NF-κB p65 pAkt (Abcam, Cambridge, UK) overnight at 4 °C. Afterward, the membranes were washed thrice using TBST and incubated with secondary antibody (LICOR, Lincoln, NE) conjugated to horseradish peroxidase for 1 h. Finally, the protein bands were detected with a two-color infrared imaging system (Odyssey, LICOR).
Histological and immunohistochemical studies
Thickness sections (5 μm) of the paraffin-embedded kidney tissues were prepared using an established procedure.Citation16 Hematoxylin and eosin (HE) staining was applied to detect the kidney injury and inflammation after UUO. Briefly, immunohistochemical analysis was performed as an established procedure as follows. Kidney sections were dewaxed and hydrated in a decreasing series of ethanol followed by antigen retrieval. Endogenous peroxidase activity was then quenched using 3% H2O2, and 5% bovine serum albumin was used to block nonspecific binding for up to 1 h. Sections were then incubated with primary antibodies [collagen-1 (COI-1) and fibronectin (FN), Abcam] at 4 °C overnight. Afterward, goat anti-rabbit secondary antibodies were added to the tissues to incubate for another 1 h, followed by the addition of diaminobenzidine (Sigma) to detect positive signals. Ten 200 × magnification random fields were selected to capture photos, which were then quantified by Image Pro-Plus 6.0 software (MediaCybernetics, Rockville, MD).
Real-time PCR
Total RNA was prepared from the kidney tissues by using Trizol reagent (Invitrogen, Waltham, MA) following the instructions of the manufacturer. First-strand cDNA was synthesized using the PrimeScriptTM RT reagent kit (TaKaRa, Japan). Initially, 2 μg of RNA extracted from the tissues was used as a template to perform one-step RT-PCR and GAPDH mouse was used as an internal control. All reactions were conducted in a 25-μL volume tube. Real-time polymerase chain reaction (PCR) was performed on the resulting cDNA with SYBR Green method and AB7500 Real-time PCR system. The threshold cycle (Ct) values of each sample were used in the 2−ΔΔCT data analysis.
Statistical analyses
Data were expressed as mean ± SD. Statistical analysis of the results was performed using one-way analysis of variance (Graph Pad Prism TM 5.0, San Diego, CA). Values of p < 0.05 were considered statistically significant.
Results
OMT prevents kidney injury and renal fibrogenesis after UUO
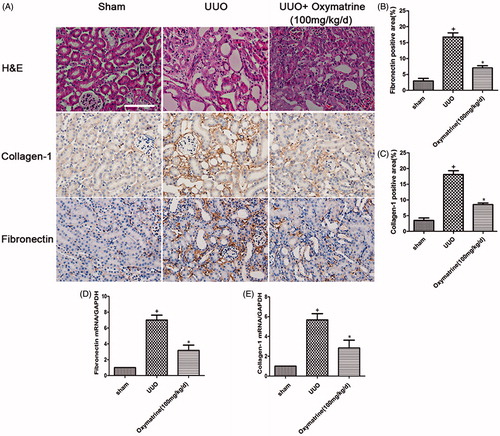
To evaluate the effect of OMT on renal tubulointerstitial fibrosis in UUO model, histologic staining methods were employed to detect kidney morphological changes after treatment. As shown in , HE staining indicated that epithelial cell atrophy, inflammatory cell infiltration, and dilated renal tubules were alleviated compared with the vehicle-treated group following OMT treatment. Consistent with the morphology of kidney injury, the deposition of COI-1 and FN is significantly diminished in 100 mg/kg/d OMT-treated group at seven days after UUO (). In addition, quantitative immunohistochemical analysis of COI-1 and FN showed marked attenuation of renal fibrogenesis in UUO model after OMT administration (). These observations were verified by real-time PCR, in which OMT significantly inhibited the expression of COI-1and FN ().
Figure 1. OMT ameliorates renal injury and interstitial matrix disposition in UUO at 7 days after surgery. (A) Representative micrographs of HE staining, immunohistochemical staining for COI-1 and FN expression, and original magnification ×200. (B, C) Quantitative analysis of FN and COI-1 for immunohistochemistry staining. (D, E) mRNA levels of COL-1 and FN in the different treated groups. +p < 0.01 versus sham group, *p < 0.01 versus UUO with vehicle-treated group. (Scale bars: 200 μm).
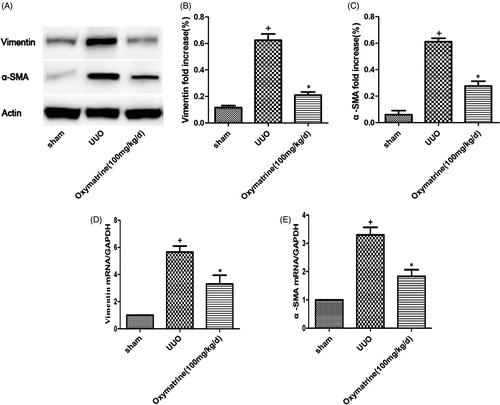
OMT prevents activation of myofibroblasts in obstructed kidney
Myofibroblasts, which are characterized by α-SMA, vimentin, and FSP-1, are pivotal fibrogenic cells in the progression of kidney fibrosis. The severity of kidney fibrosis is closely related to the accumulation of interstitial myofibroblasts. To evaluate whether OMT modulates the accumulation of myofibroblastic fibrogenic cells, the renal expression of α-SMA and vimentin was measured on postoperative day 7. As shown in , Western blot results indicated that UUO induced significant upregulation of α-SMA and vimentin, but OMT treatment markedly suppressed the α-SMA and vimentin expression levels. In addition, the results of real-time PCR also demonstrated OMT suppressing the expressions of α-SMA and vimentin, .
Figure 2. OMT suppresses activation of myofibroblasts in obstructed kidneys. (A) Western blot analysis for vimentin and α-SMA in different treatment groups. (B, C) Statistical analysis of relative expression of vimentin and α-SMA. (D, E) mRNA levels of vimentin and α-SMA in the different treated groups. +p < 0.01 versus sham group. *p < 0.05 versus UUO with vehicle-treated group.
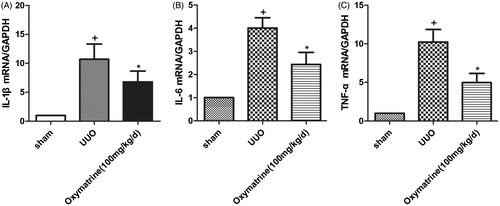
OMT blocks inflammatory cytokine release in vivo
To examine the anti-inflammatory effect of OMT on obstructed nephropathy, mRNA expression levels of TNF-α, IL-1β, and IL-6 were detected 7 days after the UUO operation. At 7 days after the UUO, a prominently increased level of proinflammatory cytokines in obstructed kidneys was observed compared with those in sham-operated mice; by contrast, OMT administration significantly attenuated the expression of these proinflammatory cytokines induced by UUO ().
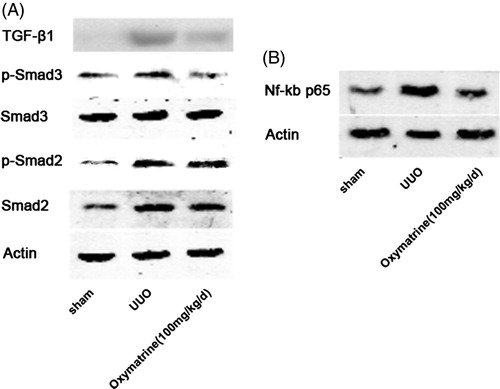
OMT inhibits activation of the TGF-β1/Smad and NF-κB-signaling pathways during UUO
To further explore the potential mechanism underlying the antifibrotic effect of OMT in UUO, this study determined the influence of OMT on the activation of TGF-β1, p-smad2, and p-smad3. shows that TGF-β1, p-smad2, and p-smad3 were markedly activated in vehicle-treated mice on day 7 after the operation. The treatment with OMT significantly suppressed the activation of TGF-β1 and smad3 phosphorylation but not smad2 phosphorylation. Furthermore, the activation of NF-κB p65 was significantly ameliorated by OMT treatment ().
Discussion
This study is the first to demonstrate that OMT ameliorated fibrosis and inflammation in the setting of fibrotic kidney after UUO. OMT markedly reduced kidney injury and interstitial ECM deposition, as well as improved renal architecture at 7 days after UUO. Moreover, OMT inhibited the activation of myofibroblasts, as evidenced by the decreased expression of α-SMA and vimentin in the obstructed kidney, probably via blocking the activation of the TGF-b1/Smad3-signaling pathways. OMT also suppressed the inflammatory responses by reducing the release of TNF-α, IL-1β, and IL-6 and inhibiting the signaling of NF-κB.
TGF-β1 has been considered as a powerful mediator in the pathogenesis of renal fibrosis.Citation17 The activation of TGF-β1 can increase the production of ECM, as well as suppress its degradation. In addition, TGF-β1 can activate myofibroblasts by activating resident fibroblasts,Citation18 inducing epithelial-to-mesenchymal transitionCitation19 or endothelial-to-mesenchymal transition,Citation20 and initiating inflammatory responses. Moreover, blocking TGF-β1 with neutralizing TGF-β1 antibodies and antisense oligonucleotides prevents the progression of renal fibrosis.Citation21 TGF-β1 executed its function mainly through the Smad-dependent and Smad-independent pathways, namely MAPK, PI3K, ERK, Rac, and GTPases.Citation22 Among these pathways, TGF-β1/Smads have been well documented and recognized to play a crucial role in the progression of renal fibrosis. Smad2 and Smad3, which are two major downstream mediators of TGF-β1, present different functional roles in renal fibrosis. Smad3 is pathogenic, and inhibition of Smad3 phosphorylation and translocation attenuates renal fibrosis.Citation23 Smad3 can directly bind to the promoter region of collagens to promote their production. At the same time, activated fibroblasts can produce more ECM, such as COI-1 and FN. On the contrary, inhibiting the activation of fibroblasts is efficient in reducing renal fibrosis in the UUO model.Citation24 Liu et al. found that OMT attenuates fibrosis via the inhibition of inducible TGF-β/Smad2/3-signaling pathway.Citation25 However, our results clearly indicated that OMT can suppress production of ECM at 7 days after the UUO via inactivated TGF-β1 and the phosphorylation of Smad3, but not Smad2, to inhibit the activation of myofibroblasts, as evidenced by the decreased α-SMA and vimentin expression levels in mice 7 days after the UUO was performed.
Certainly, inflammatory responses play an important role in the progression of various kidney diseases, including CKDs.Citation26 In the early stage of kidney injury, T lymphocytes and macrophages were applied to the damaged site to release proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α.Citation27 Activation of these factors sustains inflammation, which results in the progression of fibrogenesis. Hence, the inhibition of these cytokines shows a promising perspective in preventing kidney fibrosis. Moreover, as an inducible transcription factor, NF-κB activates enormous inflammation-associated substances to initiate and sustain inflammatory responses. These included many inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α, nitric oxide), inflammatory chemokines (monocyte chemotactic protein-1 and regulated on activation, normal T cell expressed and secreted), cell adhesion molecules (intercellular adhesion molecule 1, vascular cell adhesion molecule 1, E-selectin), a number of inducible enzymes (cyclooxygenase-2, nitric oxide synthase), growth factors and certain acute reactive proteins.Citation28,Citation29 Meanwhile, it is regulated by the positive feedback of certain cytokines (IL-1β, TNF-α), angiotensin II as well as ROS.Citation30 As such, NF-κB and its regulatory molecules together form the NF-κB-signaling pathway. NF-κB-signaling pathway is activated at an early stage of UUO and participates in the pathological formation and progression of kidney obstruction.Citation29 Blocking the nuclear translocation of NF-κB p65 attenuates mononuclear cell infiltration, proinflammatory cytokines (TNF-α, IL-1β) and chemokines (MCP-1) secretion, which dissolves inflammation,Citation31 thus attenuating tubulointerstitial fibrosis. Consistent with previous reports, the present study proved that OMT administration can inhibit p65 phosphorylation of NF-κB after UUO, and reducing the progression of inflammatory cytokines IL-1β, IL-6, and TNF-α.
In summary, this study identified direct antifibrotic effects of OMT in obstructing nephropathy. The antifibrotic mechanisms of OMT may be related to its blockage of TGFβ1-induced Smad3 phosphorylation, as well as inhibiting inflammatory responses via suppressing the activation of NF-κB at the p65 phosphorylated site. Therefore, the results present a promising compound that intervenes in the progression of renal fibrosis.
Funding information
This study was supported by the grants from the Natural Science Foundation of Hubei Province (Grant No. 2014CFC1110). This study was also supported by the grants from the Natural Science Foundation of Hubei Province (Grant No. WJ2015Z028), and the Agricultural and Social Science Development Plan of Enshi State in Hubei Province.
Disclosure statement
There is no actual or potential conflict of interest including financial, personal or other relationships with other people or organizations. All authors declare no conflict of interest related to the present work.
References
- Anothaisintawee T, Rattanasiri S, Ingsathit A, Attia J, Thakkinstian A. Prevalence of chronic kidney disease: A systematic review and meta-analysis. Clin Nephrol. 2009;71:244–254.
- Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843.
- Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl. 2014;4:2–8.
- Honda E, Park AM, Yoshida K, Tabuchi M, Munakata H. Myofibroblasts: Biochemical and proteomic approaches to fibrosis. Tohoku J Exp Med. 2013;230:67–73.
- Crisman JM, Richards LL, Valach DP, Franzoni DF, Diamond JR. Chemokine expression in the obstructed kidney. Exp Nephrol. 2001;9:241–248.
- Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82.
- Meng XM, Huang XR, Chung AC, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010;21:1477–1487.
- Liu Y, Bi T, Dai W, et al. Effects of oxymatrine on the proliferation and apoptosis of human hepatoma carcinoma cells. Technol Cancer Res Treat. 2015.
- Zhang Y, Yan R, Hu Y. Oxymatrine inhibits lipopolysaccharide-induced inflammation by down-regulating toll-like receptor 4/nuclear factor-kappa B in macrophages. Can J Physiol Pharmacol. 2015;93:253–260.
- Zhang W, Zhang J, Liu YK, et al. Cardioprotective effects of oxymatrine on isoproterenol-induced heart failure via regulation of DDAH/ADMA metabolism pathway in rats. Eur J Pharmacol. 2014;745:29–35.
- Yang J, Hou Y, Ji G, et al. Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci. 2014;52:180–190.
- Zhang S, Wu J, Wang H, et al. Liposomal oxymatrine in hepatic fibrosis treatment: formulation, in vitro and in vivo assessment. AAPS PharmSciTech 2014;15:620–629.
- Shen XC, Yang YP, Xiao TT, Peng J, Liu XD. Protective effect of oxymatrine on myocardial fibrosis induced by acute myocardial infarction in rats involved in TGF-beta(1)-Smads signal pathway. J Asian Natl Prod Res. 2011;13:215–224.
- Jiang G, Liu X, Wang M, Chen H, Chen Z, Qiu T. Oxymatrine ameliorates renal ischemia-reperfusion injury from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras. 2015;30:422–429.
- Eddy AA, Lopez-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol. 2012;27:1233–1247.
- Gustafsson OJ, Briggs MT, Condina MR, et al. MALDI imaging mass spectrometry of N-linked glycans on formalin-fixed paraffin-embedded murine kidney. Anal Bioanal Chem. 2015;407:2127–2139.
- Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transll Res. 2015;165:512–530.
- Borges FT, Melo SA, Ozdemir BC, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392.
- Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107.
- Xavier S, Vasko R, Matsumoto K, et al. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015;26:817–829.
- Border WA, Noble NA. Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int. 1998;54:1390–1391.
- Loeffler I, Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol Dial Transplant. 2014;29:i37–i45.
- Zhou Y, Mao H, Li S, et al. HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. J Am Soc Nephrol. 2010;21:598–609.
- Poosti F, Bansal R, Yazdani S, et al. Selective delivery of IFN-gamma to renal interstitial myofibroblasts: A novel strategy for the treatment of renal fibrosis. FASEB J. 2015;29:1029–1042.
- Liu L, Lu W, Ma Z, Li Z. Oxymatrine attenuates bleomycin-induced pulmonary fibrosis in mice via the inhibition of inducible nitric oxide synthase expression and the TGF-beta/Smad signaling pathway. Int J Mol Med. 2012;29:815–822.
- Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;S22–S26.
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Socf Nephrol. 2000;11:152–176.
- Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79:5353–5362.
- Esteban V, Lorenzo O, Ruperez M, et al. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:1514–1529.
- Bauge C, Beauchef G, Leclercq S, et al. NFkappaB mediates IL-1beta-induced down-regulation of TbetaRII through the modulation of Sp3 expression. J Cell Mol Med. 2008;12:1754–1766.
- Zheng X, Zhu S, Chang S, et al. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur J Pharmacol. 2013;720:147--157.