Abstract
Background: The renoprotective effect of inhibitors of renin-angiotensin system (RAS) has been identified through placebo-controlled trials. However, the effect of calcium-channel blockers (CCBs) on renal system is still controversial. Our current meta-analysis includes available evidences to compare the effect of dihydropyridine CCBs and ACEIs or ARBs on renal outcomes and mortality. We also further investigate whether CCBs can be used in combination with inhibitors of RAS to improve the prognosis of patients with chronic kidney disease (CKD). Methods and results: Electronic databases were searched up to July 2012, for clinical randomized controlled trials, assessing the effect of dihydropyridine CCBs on the incidence of end-stage renal disease (ESRD) and all-cause mortality in contrast to ACEIs or ARBs. Eight clinical trials were included containing 25,647 participants. ESRD showed significantly higher frequency with CCBs therapy compared with ACEIs or ARBs therapy, though blood pressure was decreased similarly in both groups in every trial (OR, 1.25; 95% CI, 1.05–1.48; p = 0.01). In contrast, there was no significant difference in the incidence of all-cause mortality between these two groups, though ACEIs or ARBs exhibited better renoprotective effect compared to CCBs (OR, 0.96; 95% CI, 0.89–1.03; p = 0.24). Conclusions: CCBs did not increase all-cause mortality incidence in patients with CKD though they displayed weaker renoprotective, compared to ACEIs or ARBs therapy. Our results suggest the combination of a CCB and an ACEI or ARB should be a preferable antihypertensive therapy in patients with CKD, considering their higher effect in decreasing blood pressure and fewer adverse metabolic problems caused.
Introduction
Hypertension accounts for nearly 30% of end-stage renal disease (ESRD) in the United States.Citation1 Effective blood pressure control has been well accepted as a clinical strategy to slow down the progression of chronic kidney disease (CKD) to ESRD.Citation2,Citation3 Calcium-channel blockers (CCBs) and inhibitors of renin-angiotensin system (RAS) are the most common and effective antihypertensive agents, especially the latter including angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have been proven to protect renal system in addition to those benefits resulted from lowering blood pressure alone.Citation4 Based on a large body of evidence from clinical studies, international guidelines have endorsed the opinion that inhibitors of the RAS should be the first-line antihypertensive therapy in patients with diabetic and nondiabetic nephropathy because of their protective effect in slowing down the progression of CKD to ESRD. But for many patients with hypertension as well as CKD, one ACEI or ARB is not enough to decrease blood pressure to an ideal level. Moreover, if we just increase the dose of ACEI or ARB or combine ACEI and ARB to get a nice blood pressure control, some metabolic problems especially hyperkalemia will appear, which send patients with CKD to very dangerous conditions.Citation5 Dihydropyridine CCBs, effective antihypertensive agents with little possibility to cause metabolic problems, could be used in combination with inhibitors of RAS to enhance the efficiency of lowering blood pressure for patients with CKD. However, the effects of CCBs on renal diseases drawn from current the available clinical data are quite contradictory: beneficial, neutral, even adverse.Citation6–8
To assess the action of CCBs on the progression of CKD, we undertook a systematic review and meta-analysis of clinical randomized controlled trials investigating the effects of CCBs on renal outcomes and all-cause mortality compared with ACEIs or ARBs.
Methods
Search and selection process
We did a computerized search of the Pub Med, Cochrane Library, reviews, and reference lists of relevant papers. The search strategy was supplemented manually by reviewing reference lists and querying investigators working in this field. Included studies met the following four criteria: (1) Studies had to be clinical randomized controlled trials, and the parallel-design in multicenter would be better; (2) Studies examined the effect of dihydropyridine CCBs on the progression of renal disease and all-cause mortality compared with that of ACEIs or ARBs at similar blood pressure control in CKD patients associated with hypertension and/or diabetes mellitus. If maximum tolerated doses of the blinded drug failed to effect the assigned blood pressure goal, additional drugs could be added: furosemide, doxazosin, metoprolol, clonidine, hydralazine, minoxidil and so on; (3) Progression of renal disease was assessed by using the incidence of ESRD, which is defined as the necessary for kidney transplantation, hemodialysis or a serum creatinine concentration of about 5.0 mg per deciliter, even renal death. All-cause mortality was also taken into account; (4) Studies had to have a minimum follow-up of two years and at least 100 participants. Only studies published as full-length articles in English-language journals were included.
Two reviewers (Hong-Jin Zhao and Yan Li) masked to the study authors and journals in which the studies were published, independently extracted data from published sources and determined whether the trials met the inclusion criteria. Disagreements were resolved by joint review and consensus.
Data extraction
Two reviewers, (Hong-Jin Zhao and Yan Li), independently extracted data from published sources regarding methodological features, the numbers of treated patients, mean follow-up exposure, patients’ characteristics and the occurrence of the following two outcomes: ESRD and all-cause mortality. If relevant information could not be extracted, the study authors were contacted by e-mail, with a reminder after 30 days. Uncertainties were resolved by joint review and consensus.
Statistical analysis
Meta-analyses of the trial results are presented as pooled odds ratios (ORs) and associated 95% confidence intervals (CIs) calculated using the Mantel–Haenszel method for CCBs compared with ACEIs or ARBs.Citation9 A p values less than 0.05 were considered statistically significant; all tests and CIs were two-sided. Analyses were carried out by using Review Manager statistical software (Review Manager version 5.0.21.0; The Nordic Cochrane Center, Rigs Hospitalet). The appropriation of pooling the results from individual studies was assessed using the I2 test for heterogeneity. The I2 value describes the percentage of total variation across studies due to heterogeneity rather than chance, and we considered I2 more than 50% to indicate significant heterogeneity among the trials.Citation9 All analyses were initially assessed using a fixed-effects model, and if heterogeneity across studies was observed, the analyses were repeated using a random-effects model, which includes a measure of variance in the calculation of pooled results.Citation10 Publication bias was assessed using a funnel plot of effect size against standard error.Citation11
Results
Study selection
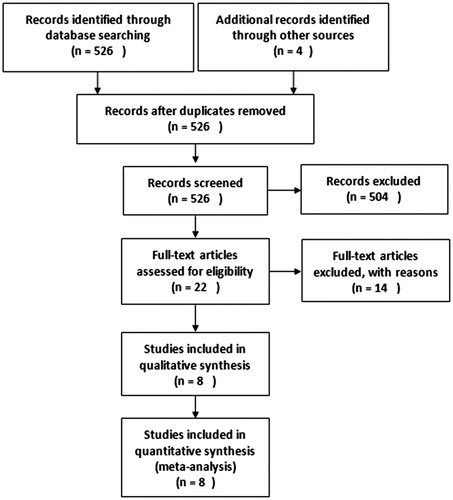
As shown in , a total of 526 potentially eligible studies were identified, of which 504 were excluded after reviewing the study titles, leaving 22 studies for a more detailed evaluation. Of these 22 studies, 14 studies were excluded for the following reasons: duplicate data in 4 studies; there were 3 studies concerning renal outcomes but no ESRD or all-cause mortalityCitation12–14; two studies were not randomized controlled trialsCitation15,Citation16; all the participants in two studies were hemodialysis patientsCitation17,Citation18; one study was about the survival only in kidney transplant patientsCitation19; the number of participants in two trials was less than 100Citation20,Citation21; Totally, eight studies were therefore included in this meta-analysis.
Study characteristics and quality
The baseline characteristics of eight included studies including a total of 25,647 patients were shown in . All included studies were randomized controlled trials (RCT) published in English, six of which were prospective RCT in multiple clinical centers with higher quality.
Table 1. Study characteristics comparing the therapeutic benefits and risks of CCBs therapy versus ACEIs or ARBs therapy.
Quantitative data synthesis and analyses
Data related to blood pressure and the outcomes of ESRD as well as all-cause mortality during follow-up is documented in .
Table 2. Study outcomes comparing the therapeutic benefits and risks of CCBs therapy versus ACEIs or ARBs therapy.
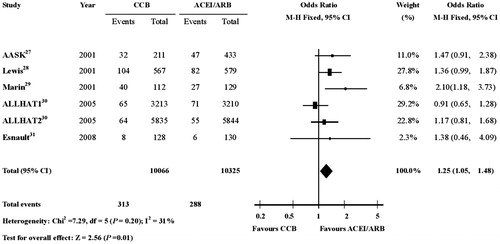
ESRD showed significantly higher frequency with CCBs therapy compared with ACEIs or ARBs therapy, though blood pressure was decreased similarly in the CCBs group and ACEIs group in every trial (OR, 1.25; 95% CI, 1.05–1.48; p = 0.01) (.
Figure 2. Risk of ESRD in patients receiving CCBs therapy or ACEIs/ARBs therapy. The meta-analysis of ESRD showed significantly more events with CCBs therapy compared with ACEIs or ARBs therapy (OR, 1.25; 95% CI, 1.05–1.48; P = 0.01). CI indicates confidence interval; OR: odds ratio; ESRD: end-stage renal disease; CCBs: calcium-channel blockers; ACEIs: angiotensin-converting-enzyme inhibitors; ARBs: angiotensin-II receptor blockers.
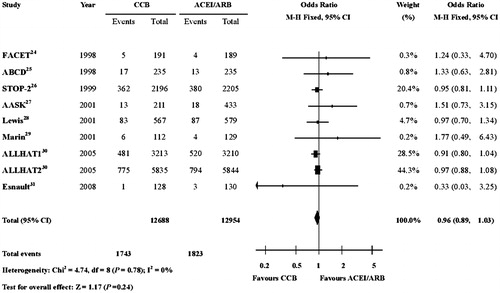
In contrast, there was no significant difference in the incidences of all-cause mortality between the two groups, though ACEIs or ARBs exhibited obvious renoprotective effect (OR, 0.96; 95% CI, 0.89–1.03; p = 0.24) (.
Figure 3. Risk of all-cause mortality in patients receiving CCBs therapy or ACEIs/ARBs therapy. There was no significant difference in the incidence of all-cause mortality between the two groups (OR, 0.96; 95% CI, 0.89–1.03; P = 0.24). CI: confidence interval; OR: odds ratio; CCBs, calcium-channel blockers; ACEIs: angiotensin-converting-enzyme inhibitors; ARBs: angiotensin-II receptor blockers.
Publication bias
This was assessed with two funnel plots, which are available from us on request. The funnel plots for ESRD and all-cause mortality during follow-up were symmetric, suggesting the absence of publication bias.
Discussion
Although similar blood pressure responses in two groups of every trial at the end of follow-up, ACEIs or ARBs reduced ESRD more effectively than CCBs. Hypertension is known as an essential risk factor for CKD and can promote the progression of CKD to ESRD. High systemic pressure transmitted to the renal microvasculature, causing increased renal intraglomerular filtration and subsequent hypertensive renal damage.Citation22 African–American study of kidney disease and hypertension (AASK) revealed that renal death happened less frequently in a relatively lower blood pressure group of patients treated with amlodipine, suggesting the renoprotective effect of dihydropyridine CCBs through antihypertensive action.Citation23 To the contrary, some experts believe that BP reduction achieved by CCBs provides the expected renoprotective effect because blockades of the “L” type Ca2+ channels lead to vasodilation of the preglomerular arteriole, rendering high renal glomerular filtration pressure that will impair the vascular wall and promote protein excretion called proteinuria that will further enhance the damage to the renal function while they reduce BP.Citation24,Citation25 On the other hand, inhibitors of RAS might effectively lower filtration pressure to improve glomerular pore size, reducing proteinuria to attenuate renal damage through inhibiting angiotensin II which has strong vasoconstrictive effect on postglomerular vasculature.Citation26 These different effects on the renal microvasculature between these two types of antihypertensive drugs may partly explain why ACEIs or ARBs can slow down the progression of CKD to ESRD much effectively than CCBs.
The accumulation of bradykinin (BK) has been confirmed as a factor to the renal protection in ACEI group.Citation27 It has been well known that ACEI can delay the degradation of BK, leading to the increment of BK throughout the body that is responsible for frequent cough in some patients treated with ACEI. However, the insulin-like effect of BK helps the improvement of insulin sensitivity, subsequently attenuating the pernicious effects of hyperglycemia to the renal microvasculature in diabetic patients.Citation27 Furthermore, beside BK, ACEI can also induce the increment of angiotensin1–7 rendering reduction of plasminogen activator inhibitor-1, which is another risk factor for the renal functionCitation28, whereas CCBs have no such additional benefits on renal outcomes.
Though ACEIs or ARBs can significantly decrease the occurrence of ESRD, showing obvious renoprotective action, there was no significant difference between inhibitor of RAS and CCBs group in all-cause mortality based on our meta-analysis. This result might due to the slightly higher BP level achieved in ACEIs or ARBs group compared with CCBs group leaded to higher occurrences of major cardiovascular events and mortality. Especially, CCBs showed their specific advantages in reducing stroke occurrence in contrast to ACEIs or ARBs, which is also an explanation for our resultCitation29 Furthermore, the risk of ACEIs or ARBs–associated anemia and especially hyperkalemia definitely contributed to mortality.Citation5
CKD patients are particularly susceptible to hypertension due to their impaired autoregulatory vasoconstriction responses of pre-glomerular arterioles.Citation22 Several studies have shown that the progression of CKD to ESRD and even mortality are mainly related to the absence of adequate blood pressure control.Citation30–32 Though inhibitors of RAS show obvious renal protective effect, mono-therapy with ACEI or ARB may not be enough to keep blood pressure in the optimal level. Simply raising the dose of ARBs or ACEIs or adopting the combination of the ACEI and ARB to enhance the blood pressure control, the serum potassium concentration would be significantly increased which may be lethal to patients, especially when the renal function was not optimal.Citation33,Citation34 Another adverse effect of high dose ACEI or ARB therapy is severe anemia, partly resulted from the reduction of angiotensin II which is known to stimulate erythropoietin in certain circumstances.Citation35 ACEIs or ARBs has been suggested as the first choice to control blood pressure in patients with CKD. However, it does not mean CCBs cannot be considered as a part of the combination of antihypertensive therapy. Shokei et al. have proved that the combination of ARB and CCB exhibited greater efficacy in preventing cardiovascular events in CKD patients compared with high dose ARB alone.Citation36 Furthermore, ACEIs or ARBs combined with CCBs has been shown to particularly reduce the risk of hyperkalemia and other metabolic problems.Citation37
In conclusion, CCBs did not increase all-cause mortality in patients with CKD though they displayed less renoprotection effect in reducing the occurrence of ESRD, compared with ACEIs or ARBs therapy. Although the long-term efficacy of the combination of a CCB and an ACEI or ARB therapy needs further confirmation, our results suggest this combined antihypertensive therapy could be a more preferable antihypertensive therapy in patients with CKD, considering its effective blood pressure controlling, an assured antiproteinuria effect and fewer adverse metabolic problems.
Funding information
This work was supported by research grants from the Natural Science Foundation of Shandong Province [2010ZRB1488].
Acknowledgements
Dr. Hong-Jin Zhao and Yan Li contributed to the data extraction from the literature, statistical analysis and the writing of this paper. Dr. Shan-Mei Liu, Xiang-Guo Sun, Min Li and Yan Hao contributed to statistical analysis and helped gather references for the manuscript. Dr. Lian-Qun Cui and Ai-Hong Wang contributed to design, direction, and supervision of data analysis and revision of the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States renal data system 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2005;45:A5–A7. S1-280.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219.
- Tozawa M, Iseki K, Iseki C, et al. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med. 1993;329:1456–1462.
- Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–2997.
- Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800.
- Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: A meta-regression analysis. Ann Intern Med. 1993;118:129–138.
- Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int. 1998;53:1559–1573.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Weinberg JM, Appel LJ, Bakris G, et al. Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169:1587–1594.
- Chan JC, Cockram CS, Nicholls MG, Cheung CK, Swaminathan R. Comparison of enalapril and nifedipine in treating non-insulin dependent diabetes associated with hypertension: one year analysis. BMJ. 1992;305:981–985.
- Kanno Y, Okada H, Yamaji Y, Nakazato Y, Suzuki H. Angiotensin-converting-enzyme inhibitors slow renal decline in IgA nephropathy, independent of tubulointerstitial fibrosis at presentation. QJM. 2005;98:199–203.
- Omae K, Ogawa T, Nitta K. Therapeutic advantage of angiotensin-converting enzyme inhibitors in patients with proteinuric chronic kidney disease. Heart Vessels.. 2010;25:203–208.
- Ziakka S, Kaperonis N, Ferentinou E, et al. Calcium channels blockers and progression of kidney disease. Ren Fail. 2007;29:1003–1012.
- Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007;2:491–500.
- Lopes AA, Bragg-Gresham JL, Ramirez SP, et al. Prescription of antihypertensive agents to haemodialysis patients: time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant. 2009;24:2809–2816.
- Hillebrand U, Suwelack BM, Loley K, et al. Blood pressure, antihypertensive treatment, and graft survival in kidney transplant patients. Transpl Int. 2009;22:1073–1080.
- Kuriyama S, Tomonari H, Tokudome G, et al. Antiproteinuric effects of combined antihypertensive therapies in patients with overt type 2 diabetic nephropathy. Hypertens Res. 2002;25:849–855.
- Chan JC, Critchley JA, Tomlinson B, Chan TY, Cockram CS. Antihypertensive and anti-albuminuric effects of losartan potassium and felodipine in Chinese elderly hypertensive patients with or without non-insulin-dependent diabetes mellitus. Am J Nephrol. 1997;17:72–80.
- Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:F1003–F1010.
- Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431.
- Gaber L, Walton C, Brown S, Bakris G. Effects of different antihypertensive treatments on morphologic progression of diabetic nephropathy in uninephrectomized dogs. Kidney Int. 1994;46:161–169.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65:1991–2002.
- Delles C, Klingbeil AU, Schneider MP, et al. Direct comparison of the effects of valsartan and amlodipine on renal hemodynamics in human essential hypertension. Am J Hypertens. 2003;16:1030–1035.
- Allard J, Buleon M, Cellier E, et al. ACE inhibitor reduces growth factor receptor expression and signaling but also albuminuria through B2-kinin glomerular receptor activation in diabetic rats. Am J Physiol Renal Physiol. 2007;293:F1083–F1092.
- Okada H, Watanabe Y, Kikuta T, et al. Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J Am Soc Nephrol. 2004;15:2404–2413.
- Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535.
- Ruilope LM, van Veldhuisen DJ, Ritz E, Luscher TF. Renal function: The Cinderella of cardiovascular risk profile. J Am Coll Cardiol. 2001;38:1782–1787.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169.
- Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174.
- Bakris GL, Siomos M, Richardson D, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. VAL-K study group. Kidney Int. 2000;58:2084–2092.
- Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. How much should we worry? Arch Intern Med. 1998;158:26–32.
- Kamper AL, Nielsen OJ. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scand J Clin Lab Invest. 1990;50:611–618.
- Shokei KM, Hisao O, Kunihiko M, et al. An angiotensin II receptor blocker–calcium channel blocker combination prevents cardiovascular events in elderly high-risk hypertensive patients with chronic kidney disease better than high-dose angiotensin II receptor blockade alone. Kidney Int. 2012;83:167–176.
- Gupta AK, Dahlof B, Dobson J, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian cardiac outcomes trial–blood pressure lowering arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–988.
- Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril versus amlodipine cardiovascular events randomized trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603.
- Estacio RO, Jeffers BW, Hiatt WR, et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652.
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish trial in old patients with hypertension-2 study. Lancet. 1999;354:1751–1756.
- Sica DA Douglas JG. The African American study of kidney disease and hypertension (AASK): New findings. J Clin Hypertens (Greenwich). 2001;3:244–251.
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860.
- Marin R, Ruilope LM, Aljama P, et al. A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19:1871–1876.
- Wright JT Jr, Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608.
- Esnault VL, Brown EA, Apetrei E, et al. The effects of amlodipine and enalapril on renal function in adults with hypertension and nondiabetic nephropathies: A 3-year, randomized, multicenter, double-blind, placebo-controlled study. Clin Ther. 2008;30:482–498.