Abstract
Lupus nephritis and renal polyarteritis nodosa (PAN) are two distinct disorders that rarely overlap. Herein, we describe a patient who was initially diagnosed with lupus nephritis based on her clinical presentation, proteinuria, hematuria, positive anti-nuclear antibody, and a kidney biopsy. A month later, the patient presented with left flank pain and weakness. A CT scan of the abdomen and pelvis showed a perinephric hematoma and the renal arteriogram revealed numerous microaneurysms within the kidney consistent with renal PAN. This case elucidates the diagnostic and management dilemmas that confront physicians taking care of patients with overlapping features of lupus nephritis and renal PAN and also points to the possible role of lupus nephritis in pathogenesis of renal PAN.
Introduction
Lupus nephritis and renal polyarteritis nodosa (PAN) both cause significant renal failure, but differ entirely in pathology. PAN has been defined as necrotizing inflammation of medium- or small-sized arteries without glomerulonephritis or vasculitis in arterioles, capillaries, or venules.Citation1 In PAN, the kidney biopsy may show arteritis and although glomeruli are usually unaffected, some glomeruli may show ischemic retraction of the tuft and sclerosis of Bowman’s capsule.Citation2 The histopathology of lupus nephritis is extremely pleomorphic, involving glomerular changes ranging from mild mesangial proliferation to diffuse proliferative nephritis with deposition of complement and immunoglobulin deposits. We present a patient with features suggestive of both lupus nephritis and renal PAN who presented a diagnostic dilemma.
Case presentation
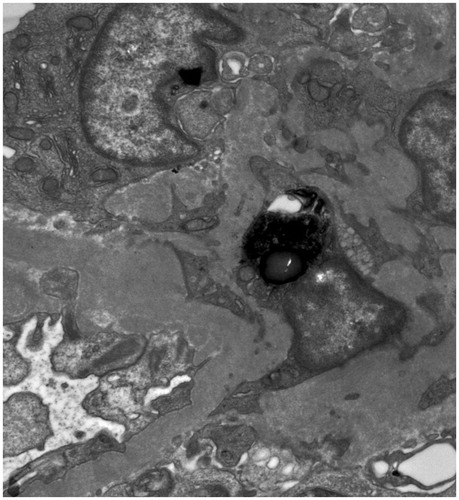
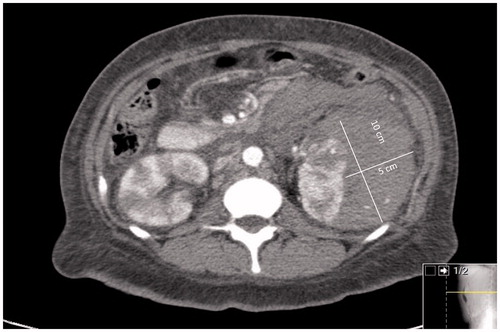
A 30-year-old African American female was referred to the Nephrology Clinic for microscopic hematuria and persistent proteinuria of 0.6–0.8 g/g creatinine on two spot samples of urine. She complained of joint pains, weight loss of 15 lbs, fatigue, but no skin rash. She had a prior history of multiple systemic infections (meningitis, cholecystitis, herpes zoster, and urinary tract infections), splenectomy for idiopathic thrombocytopenic purpura 10 years ago, and hypertension that was newly diagnosed 6 months ago. On examination, she was afebrile, and her blood pressure was 148/96 mmHg, pulse 84 beats/min, and respiratory rate 12/min. Her examination was otherwise unremarkable except for mild tenderness and swelling of the small joints of her hands. Her medications were metoprolol 100 mg twice daily and hydroxyzine 25 mg daily. Her laboratory tests showed a serum creatinine of 0.8 mg/dL, platelet count 352,000/μL, anti-nuclear antibody (ANA) > 1:640 (homogenous; normal ≤1: 40), C-reactive protein 2.17 (normal 0–0.3 mg/dL), serum complement 3 level 53 (normal 90–180 mg/dL), serum complement 4 level 17 (normal 10–40 mg/dL), and a negative serum double stranded DNA (dsDNA). She underwent a left kidney biopsy that showed mesangial hypercellularity and expansion and segmental proliferative lesions on H and E stain light microscopy (), tubuloreticular structures in endothelial cells, and ill-defined mesangial immune dense deposits on electron microscopy (). Immunofluorescence was not available as the kidney sample submitted for immunofluorescence did not contain glomeruli. A presumptive diagnosis of Class III lupus nephritis was made and she was treated with pulse prednisone with a gradual taper down to 20 mg/day. Approximately a month after her kidney biopsy, she presented to the emergency room with hypotension and left flank pain of one day’s duration. She had no history of any trauma or fall. A CT scan of the abdomen and pelvis confirmed a left perinephric hematoma (). As she continued to have hypotension and rapidly decreasing hemoglobin, a renal angiogram was performed. The angiogram showed numerous microaneurysms within the kidney consistent with the diagnosis of renal PAN (). An embolectomy coil was placed in the arterial branch that was the source of bleeding in the left kidney (). Further work-up for PAN showed normal liver function, negative hepatitis B and C serology, rheumatoid factor 46 IU/mL, negative ANCA PR3 0.2(AI) and myeloperoxidase at <0.2(AI), negative cryoglobulins, and a negative HIV screen.
Figure 1. (A) H&E stain (×200) showing increased mesangial hypercellularity in a glomerulus. (B) Silver stain (×400) showing segmental proliferative lesions in a glomerulus.
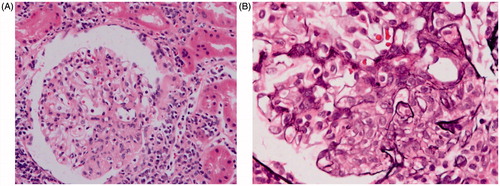
Figure 4. (A) Angiogram of the left kidney showing multiple microansurysms. (B) Coil placement in a renal artery branch feeding into the hemoatoma [white arrow].
![Figure 4. (A) Angiogram of the left kidney showing multiple microansurysms. (B) Coil placement in a renal artery branch feeding into the hemoatoma [white arrow].](/cms/asset/3c3feaa6-93fe-4809-9dab-4bf464bbe99d/irnf_a_1165075_f0004_c.jpg)
For the past 6 months, the patient has been treated with prednisone and cellcept for her diagnosis of possible lupus and renal PAN. The serum complement levels have decreased below normal, serum creatinine has slowly worsened to 2.1 mg/dL, proteinuria has increased to 1.5–2.0 g/day, and microscopic hematuria has persisted. The serum dsDNA has turned positive. Due to her prior history of multiple infections patient has not been treated with cyclophosphamide, and the repeat kidney biopsy has not pursued because of high risk of bleeding.
Discussion
In our patient, the diagnosis of lupus nephritis was uncertain on initial presentation due to normal serum creatinine and complement 4 level, absence of serum double stranded DNA, and lack of immunofluorescence information on kidney biopsy. However, this patient met the American College of Rheumatology (ACR) criteria of PAN with her history of new onset hypertension, significant weight loss, and characteristic renal angiogram showing multiple renal arterial microaneurysms. Over time, her serum creatinine and proteinuria increased, complement levels dipped to below normal range and serum double stranded DNA turned positive despite being on treatment with oral prednisone. These laboratory abnormalities can only be explained by evolution of lupus nephritis in our patient. In addition, the finding of tubulo-reticularinclusion bodies in glomerular endothelial cells and mesangial electron dense deposits on electron microscopy strongly favored a diagnosis of lupus nephritis. Hence, our patient met the criteria for both lupus nephritis and renal PAN.
Did lupus nephritis and renal PAN evolve independent of each other or did lupus cause the renal picture of PAN with micro aneursyms in our patient? We speculate that lupus might have caused renal PAN. Lupus has been known to cause different forms of vasculitis,Citation3 and although, lupus typically involves small arteries and venules, medium-sized arteritis (typical of PAN) may also occur in ∼10% patients.Citation4 The pathogenesis of PAN has been attributed to hepatitis B infection or immune complex deposits. Our patient did not have any evidence of hepatitis B infection, but immune-complex deposition in the intrinsic renal arteries as a result of lupus could have caused renal arterial wall weakening and development of microaneurysms typical of PAN. The only other case of renal PAN coexisting with lupus nephritis was reported by Bakan et al.Citation5. In the described case, digital subtraction angiogram revealed renal microaneurysms typical of PAN and a kidney biopsy showed the presence of IgG, IgA and C1q positivity with immunofluorescence examination strongly suggesting lupus nephritis, although ANA and dsDNA were both negative. Patient was treated with pulse cyclophosphamide (1 g/month 6 times) and methyl-prednisolone (1 g/month 3 times and subsequently 1 mg/kg oral maintenance) with resolution of symptoms.
The occurrence of perirenal hematoma a month after the kidney biopsy in our patient could have been a delayed bleed post-kidney biopsy or could have resulted due to a rupture of a microaneurysm in the kidney. Although we cannot rule out the former possibility, it is likely that bleeding developed from spontaneous rupture of a microaneurysm due to the long time interval between left kidney biopsy and patient’s presentation. The spontaneous non-traumatic perirenal hematoma has been named Wunderlich’s syndrome.Citation6 Although, PAN may cause peri-renal hematoma,Citation7 several other etiologies of Wunderlich’s syndrome have been reported in the literature including renal cell carcinoma, angiomyolipoma, vasculitis, cystic renal diseases, and nephritis.Citation8–11
In conclusion, lupus nephritis and PAN may coexist together causing a diagnostic dilemma, and lupus nephritis may play a role in the pathogenesis of renal PAN. The overlapping presentation of renal lupus and PAN also makes the management of these patients extremely challenging due to ambiguous clinical picture, difficulty in obtaining follow-up kidney biopsies, and unclear prognosis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Jennette JC, Falk RJ, Bacon PA, Basu N. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11.
- Colmegna I, Maldonado-Cocco JA. Polyarteritis nodosa revisited. Curr Rheumatol Rep. 2005;7:288–296.
- Ramos-Casals M, Nardi N, Lagrutta M, et al. Vasculitis in systemic lupus erythematosus: prevalence and clinical characteristics in 670 patients. Medicine (Baltimore). 2006;85:95–104.
- D'Cruz D, Cervera R, Olcay Aydintug A, Ahmed T, Front J, Hughes GRV. Systemic lupus erythematosus evolving into systemic vasculitis: a report of five cases. Br J Rheumatol. 1993;32:154–157.
- Baken A. A case of classic polyarteritis nodosa resembling lupus nephritis. Turk Neph Dial Transpl. 2014;23:169–171.
- Wunderlich CR. Handbuch der Pathologie und Therapie. 2nd ed. Stuttgart: Ebner and Seubert; 1856.
- Chao CT, Wang WJ, Ting JT. Wünderlich syndrome from lupus-associated vasculitis. Am J Kidney Dis. 2013;61:167–170.
- Ahmad M, Arora M, Reddu R, Rizvi I. Wunderlich’s syndrome (spontaneous renal hemorrhage). BMJ Case Rep. 2012; 2012. DOI: 10.1136/bcr-2012-006280.
- Zhang JQ, Fielding JR, Zou KH. Etiology of spontaneous perirenal hemorrhage: a meta-analysis. J Urol. 2002;167:1593–1596.
- May M, Seehafer M, Helke C, Stosiek P, Ehlers C, Hoschke B. [Angiomyolipoma of the kidneys as a rare cause of retroperitoneal hemorrhage. Two case reports with tuberous sclerosis Bourneville-Pringle]. Urologe A 2003;42:693–701.
- Baishya RK, Dhawan DR, Sabnis RB, Desai MR. Spontaneous subcapsular renal hematoma: a case report and review of literature. Urol Ann. 2011;3:44–46.