Abstract
Toxoplasma gondii is one of the important opportunistic pathogen among solid-organ transplant recipients and hemodialysis patients (HD). This study was aimed to detect toxoplasmosis among 50 renal transplant recipients (RTR), 135 HD and 120 healthy individuals in two cities (Kashan and Qom) that located in the center of Iran, from 2014 to 2015. Serological detection (IgG and IgM antibodies) was performed among all individuals in case and control groups. Molecular detection was performed on all IgM positive individuals or IgG positive with moderate to high (>51 IU/mL) antibody titers in HD (n = 42) and control groups (n = 21). In RTR patients, molecular detection was conducted among all seropositive or seronegative individuals (n = 50). IgG seropositivity was detected in 52% (26/50) of RTR, 63% (85/135) of HD and 33.3% (40/120) of the control group. The rate of anti-T. gondii IgG antibody was significantly elevated in RTR and HD patients than the control group (p = 0.023 and p < 0.001, respectively). IgM seropositivity was only detected in one HD patient. T. gondii DNA was detected in 12% (6/50) of RTR and 7.1% (3/42) of HD patients. The results of this study suggested that the screening of toxoplasmosis should be given greater consideration among RTR and hemodialysis patients.
Introduction
Immunocompromised patients are at risk of several infectious diseases.Citation1 Immunosuppressive therapies have reduced the incidence of an organ recipient rejection, but increased susceptibility to opportunistic infections.Citation2,Citation3 Toxoplasmosis is one of the most important infectious agents in immunocompromised patients.Citation4,Citation5 The infection usually occurs by consuming undercooked meat containing Toxoplasma gondii tissue cysts or drinking contaminated water with oocysts shed in the feces of cats.Citation6 In transplant recipient individuals, the infection usually transmitted from a Toxoplasma—seropositive donor to a Toxoplasma—seronegative recipient. Toxoplasmosis is usually latent (asymptomatic) in immunocompetent individuals, but the infection is associated with life threatening outcomes in immunocompromised patients.Citation7 Importantly, reactivation of latent toxoplasmosis in seropositive recipients can cause severe disease with fatal outcome.Citation8 Also, the risk of infection may increase when both the donor and recipient are Toxoplasma-seropositive.Citation9 In hemodialysis patients, disturbances of the immune system potentially increased susceptibility to opportunistic infections, including toxoplasmosis.Citation10–12 In this regard, several cases of fatal and severe toxoplasmosis have been reported in kidney transplant recipients.Citation13–20 Hence, early diagnosis of toxoplasmosis is important for successful treatment of this infection.
In Iran, the overall seroprevalence rate of toxoplasmosis reported a range between 33% and 45% among healthy populations and 43–56% among immunocompromised patients (see recent systematic reviewsCitation21,Citation22). Different studies also demonstrated significantly higher seroprevalence rates of toxoplasmosis in hemodialysis patients than healthy subjects in Iran.Citation23–25
Iran is one of the pioneering countries in hemodialysis and renal transplantation.Citation26,Citation27 While the first case of renal transplantation took place in 1967, the number of transplantation has been dramatically increased until now.Citation27–29 Hence, increase in surveillance and prevention of secondary infections is needed for immunocompromised patients to prevent life threatening outcome.
Although different cases of toxoplasmosis have been reported in renal transplant recipients (RTR),Citation13–20 but few serological and molecular studies of toxoplasmosis have been conducted among RTR and hemodialysis patients.Citation30 Hence, the aim of this study is serological and molecular detections of toxoplasmosis among kidney transplant recipients, hemodialysis patients and healthy individuals in Kashan and Qom cities, which located in the central region of Iran.
Materials and methods
Study population
This study was performed among 135 HD, 50 RTR and 120 healthy subjects. The samples were collected from Kashan (Shaheed Beheshti and Akhavan hospitals) and Qom (Vali-asar hospital) cities in the center of Iran, from 2014 to 2015. This study was approved by the Ethical Committee of Kashan University of Medical Sciences, Iran.
Serological evaluation
Five milliliters of venous blood samples were collected from case and control groups. IgG and IgM antibodies to T. gondii were detected by the enzyme-linked immunosorbent assay, with a commercial ELISA kit (Pishtaz Teb, Tehran, Iran) according to the manufacturer’s instructions. The diagnostic criteria of IgG and IgM antibodies were defined as the upper limit of the standard 11 IU/mL (cutoff).
Molecular detection
Molecular detection was conducted on Buffy coat samples of all seropositive or seronegative RTR (n = 50). In hemodialysis and control groups, molecular detection was performed on all IgM positive individuals or IgG positive with moderate to high (>51 IU/mL) antibody titers (). DNA was extracted from Buffy coat samples using DNG plus kit (Sinaclon, Tehran, Iran) according to the manufacturer’s protocol. Positive DNA was extracted from the RH strain of T. gondii (prepared from the Department of Parasitology at Tarbiat Modares University). PCR was carried out using a pair of T. gondii primers targeting the GRA6 gene, which amplify a 344 bp fragment of T. gondii genome.Citation31 First round PCR was performed using external primers GAR6-F1 (5′-ATTTGTGTTTCCGAGCAGGT-3′) and GAR6-R1 (5′-GCACCTTCGCTTGTGGTT-3′). Nested-PCR was performed with internal primer GAR6-F2 (5′-TTTCCGAGCAGGTGACCT-3′) and GAR6-R2 (5′-TCGCCGAAGAGTTGACATAG-3′).Citation31 Amplification was performed in a final volume of 20 μL reaction mixtures containing 10 pmol of each forward and reverse primers, 10 μL of 2× Taq DNA polymerase Master Mix with 1.5 mM MgCl2 (Cat. no. A170301, Ampliqon, Odense, Denmark), 4 μL of template DNA and 4 μL of distilled water. The PCR conditions were 5 min at 94 °C (initial denaturation), followed by 35 cycles of 94 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 30 s and final extension at 72 °C for 5 min. The nested-PCR was conducted with the same reaction conditions of the first round; except an annealing temperature of 57.5 °C was used. One microliter of first round PCR product was used as the template for nested-PCR. For each reaction, a negative control (double distilled water) and a positive control (DNA extracted from the RH strain of T. gondii) were included. Nested-PCR products along with a 100-bp DNA ladder were electrophoresed in 1.5% agarose gel stained with ethidium bromide and visualized under ultra-violet trans-illumination.
Table 1. Serological findings among RTR, HD and control groups.
Statistical analysis
The data were analyzed by chi-square (exact sig-2-sided), Fisher exact tests, t-test, Leven test and Kolmogorov–Smirnov test by SPSS version 11.5 (SPSS Inc., Chicago, IL).
Results
The mean age of the RTR, HD and control groups were 47.8 ± 12.8, 58.6 ± 16.1 and 52.76 ± 17.03 years, respectively, which the difference was not statistically significant. According to the results, IgG seropositivity was detected in 52% (26/50), 63% (85/135) and 33.3% (40/120) of RTR, HD patients and control group, respectively (). Statistical analysis revealed the rate of anti-T. gondii IgG antibody was significantly higher in RTR and HD patients than the control group (p = 0.023 and p < 0.001, respectively). T. gondii IgM antibody was not detected in the RTR patients and the control group, but detected in one HD patient (). T. gondii DNA was detected in 12 (6/50) of RTR, 7.1% (3/42) of HD patients and none of the control group ( and ).
Figure 1. Nested-PCR products of the GRA6 gene. T. gondii positive samples give a 344-bp band. M: 100 bp DNA marker; lane 1 positive control, lane 3 negative control, lanes 2, 4–11 positive samples.
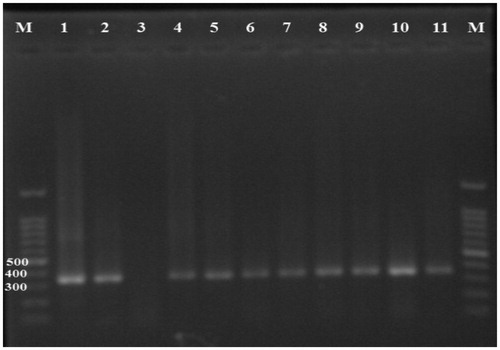
Table 2. Serological findings and clinical symptoms among PCR positive patients (hemodialysis and transplant).
Among nine PCR positive patients, six patients were belonging to the RTR group and three of them were from the HD group ().
Discussion
Toxoplasmosis is one of the infectious causes of morbidity and mortality among solid-organ transplant recipients and hemodialysis patients.Citation8,Citation30 Several cases of fatal and severe toxoplasmosis have been reported in RTR.Citation13–20 Moreover, higher seroprevalence of toxoplasmosis has been reported in hemodialysis and chronic renal failure patients.Citation12,Citation23,Citation25,Citation32 The diagnosis of toxoplasmosis is based on serology. If an individual was IgM or IgM + IgG positive, the patient has an active infection. IgG positive alone indicated previous infection. However, in the case of IgG positive with high titers, additional tests should be conducted to indicate an active infection or not.Citation33,Citation34 Therefore, in the current study, we conducted nested-PCR in IgG positive HD patients and healthy controls with moderate to high IgG titers (>51 IU/mL). But, PCR was performed on all seropositive or seronegative RTR (n = 50).
In our study, T. gondii IgG seropositivity was significantly increased in RTR (52%, p = 0.023) and HD patients (63%, p < 0.001) than the control group (33.3%) (). Moreover, IgM seropositivity was detected in one HD patient. It is of interest that this patient was IgG negative (). In recent studies, T. gondii IgG seropositivity was detected in 60% of HD patients and 40% of the healthy control group (P > 0.05) in Babol (North Iran).Citation23 Ebrahim Zadeh et al.Citation24 detected T. gondii IgG antibody among 56.7% of HD patients and 29.7% of the control group (p = 0.036) in Zahedan (South-East Iran). T. gondii IgM antibody was also detected in 13.5% of the case and none of the control group.Citation24 Maraghi et al.Citation25 found that T. gondii IgG and IgM antibodies were significantly elevated in HD patients than the control group. According to the results,Citation25 T. gondii IgG was detected in 40.67% and 26% of the case and control groups, respectively. T. gondii IgM seropositivity was detected in 8.6% of HD patients and none of the control group.Citation25
The diagnosis of active T. gondii infection by molecular methods is more sensitive than serological tests.Citation33,Citation34 In the current study, T. gondii DNA was detected in 6 of 50 (12%) RTR patients. Among them, only three patients were IgG positive and none of them were IgM positive. Also, T. gondii DNA was detected in three HD patients, which two of them had high IgG antibody titers, and one of them was IgM positive (). These results indicated the importance of molecular diagnostic tests for detection toxoplasmosis in immunocompromised patients. In this regard, Saki et al.Citation12 detected T. gondii DNA in 1.4% (4/280) of HD patients and none of healthy individuals. They also detected 29.3% and 26% IgG seropositivity and 7.9% and 4% IgM seropositivity among HD patients and control group, respectively (p < 0.05).
In conclusion, our results revealed that renal transplant recipients and hemodialysis patients are at high risk for toxoplasmosis. Therefore, programs for prevention, screening and treatment of toxoplasmosis should be given greater consideration among these patients.
Funding information
The authors acknowledge the vice chancellor in research affairs of Kashan University of Medical Sciences for financial support (Grant no. 9328).
Disclosure statement
The authors report no conflicts of interest.
References
- Fishman JA. Opportunistic infections—Coming to the limits of immunosuppression? Cold Spring Harb Perspect Med. 2013;3:a015669.
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614.
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729.
- Cohen SN. Toxoplasmosis in patients receiving immunosuppressive therapy. JAMA. 1970;211:657–660.
- Cohen BA, Stosor V. Opportunistic infections of the central nervous system in the transplant patient. Curr Neurol Neurosci Rep. 2013;13:1–12.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976.
- Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7:1–17.
- Derouin F, Pelloux H. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect. 2008;14:1089–1101.
- Castagnini M, Bernazzali S, Ginanneschi C, et al. Fatal disseminated toxoplasmosis in a cardiac transplantation with seropositive match for Toxoplasma: should prophylaxis be extended? Transplant Immunol. 2007;18:193–197.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Basic science and dialysis: disturbances of acquired immunity in hemodialysis patients. Semin Dialysis. 2007;20:440–451.
- Alter MJ, Lyerla RL, Tokars JI, Miller ER, Arduino MJ. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR. 2001;50:1–43.
- Saki J, Khademvatan S, Soltani S, Shahbazian H. Detection of toxoplasmosis in patients with end-stage renal disease by enzyme-linked immunosorbent assay and polymerase chain reaction methods. Parasitol Res. 2013;112:163–168.
- Clissold R, Bingham C. Late presentation of toxoplasmosis in renal transplant recipients. NDT Plus 2010;3:480–482.
- Vaughan L, Wenzel R. Disseminated toxoplasmosis presenting as septic shock five weeks after renal transplantation. Transpl Infect Dis. 2013;15:E20–E24.
- Nasser Q, Power R, Eng M, Hickey D, Little D. Toxoplasmosis after a simultaneous pancreas and kidney transplantation. Transplant Proceed. 2004;36(9):2843–2844.
- Wulf M, Van Crevel R, Portier R, et al. Toxoplasmosis after renal transplantation: Implications of a missed diagnosis. J Clin Microbiol. 2005;43:3544–3547.
- Renoult E, Georges E, Biava M-F, et al. Toxoplasmosis in kidney transplant recipients: Report of six cases and review. Clin Infect Dis. 1997;24:625–634.
- Jeong Y-H, Park J-W, Do J-Y, et al. Toxoplasmosis after kidney transplantation. J Korean Soc Transplant. 2013;27:185–189.
- Segall L, Moal MC, Doucet L, Kergoat N, Bourbigot B. Toxoplasmosis-associated hemophagocytic syndrome in renal transplantation. Transpl Int. 2006;19:78–80.
- Rogers N, Peh CA, Faull R, Pannell M, Cooper J, Russ G. Transmission of toxoplasmosis in two renal allograft recipients receiving an organ from the same donor. Transplant Infect Dis. 2008;10:71–74.
- Daryani A, Sarvi S, Aarabi M, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: A systematic review and meta-analysis. Acta Tropica. 2014;137:185–194.
- Ahmadpour E, Daryani A, Sharif M, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. 2014;8:1503–1510.
- Bayani M, Mostafazadeh A, Oliaee F, Kalantari N. The prevalence of Toxoplasma gondii in hemodialysis patients. Iranian Red Crescent Med J. 2013;15:e5225.
- Ebrahim Zadeh A, Bamedi T, Etemadi S, Shahrakipour M, Saryazdipour K. Toxoplasmosis as a complication of transfusion in hemodialysis patients. Iran J Ped Hematol Oncol. 2014;4:22–25.
- Maraghi S, Yadyad MJ, Sheikhi M, Shamakhteh F, Latifi SM. Study the anti-Toxoplasma antibodies (IgG and IgM) in hemodialysis patients of Abadan and Khoramshahr cities southwest Iran in 2011 using ELISA. Jundishapur J Microbiol. 2013;6:e7113. DOI: 10.5812/jjm.7113.
- Aghighi M, Heidary Rouchi A, Zamyadi M, et al. Dialysis in Iran. Iran J Kidney Dis. 2008;2:11–15.
- Ghods AJ. Renal transplantation in Iran. Nephrol Dial Transplant. 2002;17:222–228.
- Mahdavi-Mazdeh M. The Iranian model of living renal transplantation. Kidney Int. 2012;82:627–634.
- Ghods AJ, Savaj S. Iranian model of paid and regulated living-unrelated kidney donation. Clin J Am Soc Nephrol. 2006;1:1136–1145.
- Fernàndez-Sabé N, Cervera C, Fariñas MC, et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: A matched case–control study. Clin Infect Dis. 2011;54:355–361.
- Khan A, Su C, German M, Storch G, Clifford D, Sibley LD. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J Clin Microbiol. 2005;43:5881–5887.
- Demirtaş F, Yalçin Ş, Yaman O, Tokgöz B, Utaş C, Şahin İ. Anti-Toxoplasma gondii antibodies in haemodialysis patients with chronic renal failure. Yonsei Med J. 2003;44:288–292.
- Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–296.
- Liu Q, Wang Z-D, Huang S-Y, Zhu X-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors. 2015;8:292. DOI: 10.1186/s13071-015-0902-6.
