Abstract
Animals in the aquatic environment carry a particular bacterial flora that is a reflection of their environment. In the present study the level of total heterotrophic bacterial (THB) populations observed in the gut of crabs simply reflected the THB in the water and sediment of the environments from which the crabs were collected. Crabs were collected from the different areas, i.e. neritic, mangrove and oyster zones, of the Vellar estuarine environment of the southeast coast of India. THB populations were high in the water and sediment in the mangrove and neritic zones. Among the three stations, less bacterial populations were observed in the water, sediment and the gut of the crabs in the oyster zones due to the constant disturbance of wave action from the estuarine mouth region. Bacteria belonging to the genera Pseudomonas and Vibrio were found to be higher in all the three different stations. In the mangrove region, Bacillus was the dominant genus in sediment samples.
Introduction
The gut serves as the natural habitat for innumerable bacteria, some are beneficial to the host and others are harmful. The primary function of the gut is to take up water and nutrients. The specific role of the resident colonic microbiota in digestion is to ferment the substances provided in the diet (e.g. dietary fibre) that cannot be digested by the host in the small intestine. The role of microbiota as food, symbiont or competitor in the nutrition of marine invertebrate detritivores is not clearly understood. The gut microbiota of aquatic invertebrates highlights the questions and processes that merit acquisition of an understanding of the role of gut microbes in the physiology of host invertebrates and nutrient dynamics of aquatic systems. A number of food products have been developed that can modify the intestinal microbiota and possibly benefit health. These contain probiotics, prebiotics and symbiotics (a combination of probiotics and prebiotics). The role of gut microbiota in the digestion of food of domestic herbivorores is well known. The gut microbiota also plays an important role in the susceptibility of marine deposit feeders. The biodiversity and in situ abundance of the gut microbiota of abalone (Haliotis discus hannai) as determined by culture-independent techniques have been investigated (Citation1,Citation2). The gut microbiota composition and the non-specific humoral and cellular immune responses in rainbow trout Oncorhynchus mykiss have been studied (Citation3). The role of gut microbiota and probiotic effects in irritable bowel syndrome have also been studied (Citation4).
The gut serves as the natural habitat for a great number of bacteria – some beneficial to the host, others harmful. The latter category includes clostridia, sulphate reducers and proteolytic bacteria, which are responsible for producing toxins that can cause diarrhoea, mucosal invasion and carcino-genesis. Saccharolytic species, primarily bifid bacteria and lactobacilli, are the main health-promoting bacteria and are thought to be important barriers to disease (Citation5).
The amylase-producing ability of intestinal bacteria in one mangrove crab and seven fish species has been determined (Citation6). Bacillus Coryneforms, Enterobacteriaceae, Flavobacterium, Micrococcus, Pseudomonas and Vibrio sp. were found in the intestinal tracts of Japanese flounder (Citation7). It has also been reported by several workers that fish of marine origin harbor Vibrio sp. as the predominant intestinal microbiota (Citation8). However, such information on the gut microbiota of estuarine crabs and their environment has not been studied in detail, hence the present study was carried out.
Material and methods
Samples were collected from the different areas, namely neritic region, mangrove region and oyster regions, of the Vellar estuary () during pre and post monsoon seasons in 2006–2007 (Lat 11° 29′ N; 79°46′ E). The crab species were picked and transferred to sterile polythene bags using sterile forceps. All the samples were transported immediately to the laboratory and subjected to various analyses. Water sample were collected from all the three stations using sterile bottles (approximately 100 ml from each site). Sediment samples were also collected from the same stations. Approximately 100 g of the sediment were transferred aseptically to fresh polythene bags using sterile spatula.
Enumeration of Total Heterotrophic Bacterial (THB) population
The microbial loads were analysed by a serial dilution plating technique using Zobell Marine agar medium (Citation9). After homogenizing the collected water sample, 1 ml of water sample was pipetted out using a sterile pipette into a 99 ml blank and shaken well. From this, 1 ml was pipetted out and added to the 9 ml blank, likewise the serial dilutions were used as inocula. For a sediment sample, 1 g of sediment from each sample was transferred aseptically to a 99 ml blank. The contents were homogenized for 10 min.
From this, 1 ml was transferred aseptically to a 9 ml blank and mixed thoroughly. Similarly serial dilutions were made and used as inocula. For gut analysis, the digestive system was dissected out aseptically using sterile scissors and forceps. Guts were homogenized in a tissue homogenizer and transferred to a 99 ml blank, from the suspensions, 1 ml of sample was pipetted out and added to a 9 ml blank; likewise serial dilutions were made. For all the samples triplicate plates were inoculated at a temperature of 28 ± 1°C for 2–7 days and the colonies were counted. The microbial load was counted and expressed as the number of colony forming units (CFU). The bacteria were identified to generic level (Citation10,Citation11).
Results
THB load in water samples
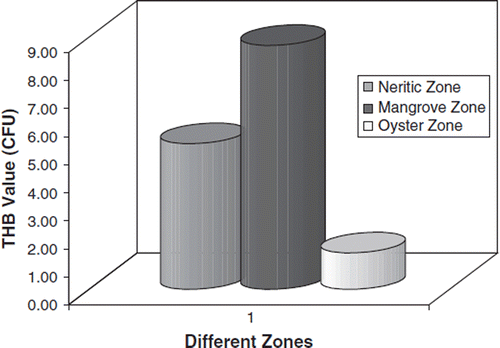
The THB count in water from three different estuarine environments is shown in . Among the three zones, a high bacterial load in water was recorded in the mangrove region (8.70×105) followed by the neritic zone (5.20×103) and the oyster zone (1.30×107). The oyster zone, which had much wave influence, showed much lower counts of THB than the mangrove region.
THB load in sediment samples
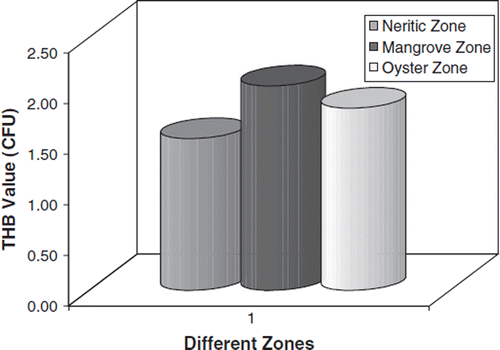
The THB count in sediment collected from three different environments is shown in . For the sediment, a high THB load was noted in the mangrove region (2.02×104) and a lower bacterial count (1.50×104) in the neritic zone region.
THB load in the gut of Scylla serrata
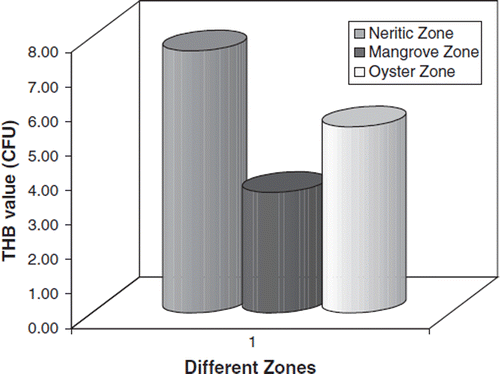
The THB counts in the gut of S. serrata collected from three different stations are shown in . S. serrata collected from the three different regions showed a high THB load (7.80×105) in the gut of crabs collected from the neritic zone.
Comparison of different crabs for gut microbiota
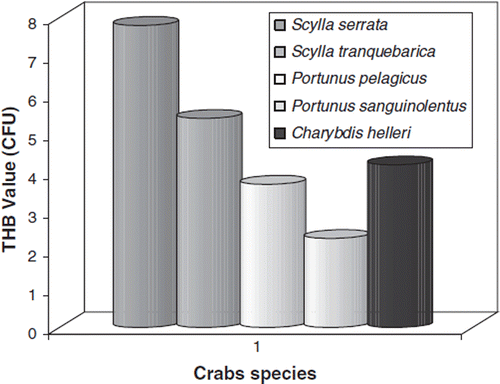
Comparison of the THB count in the gut region of five economically important crabs is shown in . The THB load varied in the guts of crab species investigated. The highest THB load was noted in the gut of mud crabs Scylla serrata (7.80×105) and S. tranquebarica (5.40×105) followed by swimming crab Charybdis helleri (4.20×105), mosac crab Portunus pelagicus (3.70×105) and three eye spot crab P. sanguinolentus (2.30×105). The count in other crabs was shown to be much lower than in the mud crab species. The lowest count was noted in the gut of P. sanguinolentus (2.30×105). The bacterial genera isolated from the water, crabs and sediment samples were Bacillus, Micrococcus, Coryneforms, Vibrio, Pseudomonas, Aeromonas, Achrobacter, Flavobacterium and Enterobacterium. The bacterial flora collected from the gut region of crabs is a reflection of their environment.
Discussion
The phylogenetic history of decapods plays an important role in the gross structure of the foregut. It is clear that bacterial species in the gut can influence the health and robustness of the host. Extreme examples of the influence of the gut flora include the negative effects of pathogenic organisms and, in contrast, the total reliance that ruminants have on their gut flora for the assimilation of organic carbon from the environment (Citation12). More subtle interactions have usually been much less characterized and understood but are, nevertheless, important and have been shown to impact on the health and growth of the host (Citation13).
The influence of the gut flora on the host is clearly of great interest in aquaculture, particularly where poor productivity and/or stock losses are widespread (Citation1). Within marine and other aquatic marine animals, the colonization of the digestive system by microorganisms is influenced by a number of both host-related and non-host-related factors (Citation14).
Within a natural environment, conditions may lead to the development of stable populations of gut flora, which may represent the normal flora of the host animal (Citation15). The degradation of litter to particulate organic matter and to fine detritus is a complex process of ecological energetic. The microorganisms convert substances such as cellulose and lignin present in the mangrove leaves into digestible matter, which is utilized by the animal communities (Citation16,Citation17). In general, the initial decomposition is carried out by microorganisms like bacteria, followed by higher organisms such as crabs, and the energy is transferred to higher trophic levels.
Animals in the aquatic environment carry a particular bacterial flora, which is a reflection of their environment. The levels of THB observed in the guts of crabs simply reflected the THB in the water and sediment of the environments from which the crabs were collected. But the sediment and water of the marine environment carry lower populations of bacteria than the alimentary tracts of animals (Citation18).
The digestive tracts of most of the marine animals contain a commensal microbiota (Citation19). The role of the gut microbiota in the digestion of food in herbivorous is well known. The gut microbiota plays a vital role in the digestive process, growth and disease susceptibility of marine deposit feeders (Citation20).
Bacteria living in the gut region have the ability to digest the carbohydrates (Citation21). Further, the midgut of crabs supports good growth of proteolytic bacteria (Citation22). Moreover, in comparison with the diverse bacterial population that has been suggested to occur in healthy individuals (under normal conditions) (Citation23), the digestive system of unhealthy or diseased prawns may be found to support a gut flora where only one or two bacterial species predominate (Citation24). In the present study, a higher level of bacterial load was observed in the gut of S. serrata and a lower level in C. helleri and P. pelagicus. Thus the bacterial load varied with feeding habits. S. serrata has a high level of gut bacterial flora due to the carnivorous feeding habitat of the mollusc Buillia vittata.
Generally, the tropical mangrove ecosystem is endowed with a high bacterial load due to the continuous shedding of foliage into the water and subsequent decomposition (Citation25). THB populations were high in the water and sediment in the mangrove region and neritic zone. Among the three stations studied, smaller bacterial populations were observed in the water, sediment and the gut of the crabs in the oyster region due to the constant disturbance of wave action from the estuarine mouth region. Bacteria belonging to the genera Pseudomonas and Vibrio were found to at higher levels in all the three different stations. In the mangrove region, Bacillus was the dominant genus in sediment samples. In conclusion, crabs in the various estuarine environments carry a particular bacterial flora, which is a reflection of their environment.
Acknowledgements
The authors are grateful to the Director of CAS in Marine Biology and authorities of Annamalai University, and to the Centre for Marine Living Resources and Ecology (CMLRE), Government of India, Cochin, for the financial support, and staff members of ENVIS centre for their keen interest and encouragement.
References
- Ravichandran S, Kannupandi T. Total heterotrophic bacterial load in decomposing mangrove litter and gut of crabs. Asian J Microbiol. 2005;7:861–4.
- Tanaka R, Ootsubo M, Sawabe T, Ezura Y, Tajima K. Biodiversity and in situ abundance of gut microflora of abalone (Haliotis discus hannai) determined by culture-independent techniques. Aquaculture. 2004;241:453–63.
- Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H, . Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol. 2004;102:379–88.
- Fanigliulo L, Comparato G, Aragona G, Cavallaro L, Iori V, Maino M, . Role of gut microflora and probiotic effects in the irritable bowel syndrome. Acta Biomed. 2006;77:85–90.
- Salminen S, Bouley S, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, . Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998; 80(Suppl 1):S147–71.
- Sugita H, Kawaskai J, Kumazarwa J, Deguchi Y. Production of amylase by the intertidal bacteria of Japanese coastal animals. Lett Appl Microbiol. 1996;23:174–8.
- Sugita H, Okano R, Suzuki Y, Iwai D, Mizukami M, Akiyama N, . Antibacterial abilities of intestinal bacteria from larval and juvenile Japanese flounder against fish pathogens. Fisheries Science. 2002;68:1004–11.
- Sugita H, Miyajima C, Deguchi Y. The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture. 1991;92:267–76.
- Zobell CE. Marine microbiology. Walthum, MA: Chronica Botanica Co; 1946. 240.
- Krieg NR, Holt JG .Bergey's manual of determinative bacteriology, 9th Baltimore: Williams and Williams Co. 1984. 220–9.
- Harley JP, Prescott LM. Laboratory exercises in microbiology. Iowa: Wm C. Brown; 1990.
- Kennedy DG, Young PB, McCaughey WJ, Kennedy S, Blanchflower WJ. Rumen succinate production may ameliorate the effects of cobalt-vitamin B-12 deficiency on methylmalonyl CoA mutase in sheep. J Nutr. 1991;121:1236–42.
- Phillips NW. Role of different microbes and subtrates as potential suppliers of specific, essential nutrients to marine detrivores. Bulletin of Marine Sciences. 1984;35:283–98.
- Harris JM. The presence, nature and role of gut microflora in aquatic invertebrates. Microb Ecol. 1993;25:195–31.
- Van der Waiij D. Mechanisms involved in the development of the intestinal microflora in relation to the host organism; consequences for colonization resistance. Hormaeche CE, Penn CW, Smyth CJ. Molecular biology of bacterial infection. Cambridge: Cambridge University Press; 1992. 1–12.
- Ravichandran S, Kannupandi T. Biochemical changes in decomposing leaves and crabs of Pichavaram mangroves. Biochem Cell Arch. 2004;24:79–88.
- Ravichandran S, Anthonisami A, Kannupandi T, Balasubramanian T. Leaf choice of Herbivorous mangrove crabs. J Fish Aquatic Sci. 2007;1:26–30.
- Yanagita T, Ichikava T, Tsugi T, Kamata K, Ito K, Sasaki M, . Two trophic groups of bacteria Oligotrophs and eutrophs; their distribution in fresh water and seawater areas in the central northen Japan. J Gen Appl Microbiol. 1978;24:59–88.
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33.
- Lapez GR, Livinton JS, Slobodkin LB. The effect of grazing by the detrivore Orchestia grillus on spartina litter and its associated microbial community. Oecologia. 1977;30:111–27.
- Galli DR, Giese AC. Carbohydrate digestion in herbivores snail Tegula Funbralis. J Exp Zool. 1959;140:415–40.
- Vankateswaran K, Sethuramalingam S, Natarajan R. Gut microflora of some edible crabs from Portonovo coast. Indian J Mar Sci. 1981;10:399–401.
- Gomez-Gil B, Tronmayen L, Rogue A, Turnbull JF, Inglis V, Guerraflores AL, . Species of Vibrio isolated from hepatopancreas, haemolymph and digestive tract of a population of healthy juvenile Penaeus vannamei. Aquaculture. 1998;163:1–9.
- Song YL, Cheng W, Wang CH. Isolation and characterization of Vibriodamsela infectious for cultured shrimp in Taiwan. J Inverteb Pathol. 1993;61:24–31.
- Palaniappan R, Krishnamurthy K. Heterotrophic bacteria of near shore waters of Bay of Bengal and the Arabian Sea. Indian J Mar Sci. 1985;14:113–4.