Abstract
Background and aim: Ischemia-reperfusion (I/R) in the intestines activates leukocytes and reactive oxygen species (ROS) and leads to lipid peroxidation and DNA damage. Rosehips have a high content of polyphenols and might prevent lipid peroxidation. Some Lactobacillus species are capable of degrading polyphenols to simpler and non-toxic constituents, sometimes with enhanced antioxidative capacity. Methods: A mixture of eight polyphenol active Lactobacillus strains (LAB) were administered in feed together with rosehips of Rosa canina (RC) or Rosa pimpinellifolia (RP) to mice to clarify the influence on I/R-induced injury of the intestinal tract. I/R injury was induced by holding the superior mesenteric artery clamped for 30 min followed by reperfusion for 240 min. Tissue and content from colon and cecum were analyzed. Results: Administration of LAB or RP significantly decreased malondialdehyde (MDA) levels in colonic tissue (p=0.007 and p=0.035, respectively). RC contained significantly higher concentrations of monomer and dimer aglycones, glycosides, and catechin than RP, while cyanidin-3-O-glycoside was significantly higher in RP. There was a tendency towards higher values of phenolics in the mice fed either of the two rose species in combination with bacterial mixture than the mice fed the rose species alone. Total antioxidative capacity and total phenolic content were higher in the groups fed rosehips in combination with LAB than rosehips alone, although these differences were not significant. Conclusion: LAB showed metabolic activity towards polyphenols in rosehips. There is an indication that LAB together with rosehips, especially R. pimpinellifolia, may prevent and suppress I/R injury in the intestines.
Introduction
The gut is a complex and dynamic ecosystem with a high bacterial load. It is also an immune organ, represented by the gut-associated lymphoid tissue or GALT (Citation1). The intestinal mucosa has a large surface area and separates the tissue from the luminal content. Dysfunction of the mucosal barrier can result in translocation of bacteria, for example, endotoxin-containing bacteria such as Enterobacteriaceae out to extraintestinal sites. This can lead to sepsis, shock, and multiple organ failure (MOF) (Citation2). Gastrointestinal (GI) ischemia followed by reperfusion (I/R) causes physical damage to the mucosal barrier and is associated with increased permeability and bacterial translocation (Citation3). I/R is encountered in trauma, strangulated bowel, vascular surgery, and hemorrhagic shock, and results in local and systemic inflammation (Citation4). Proinflammatory mediators released during I/R injury are involved in attraction, adhesion, and activation of neutrophils at the sites of injury. Activated neutrophils produce proteases and reactive oxygen species (ROS), which are associated with membrane destruction by lipid peroxidation, oxidation of proteins and enzymes, and damage to DNA (Citation5). I/R models are used for studies of oxidative stress, since ROS released during the procedure play a crucial role in tissue injury and inflammation. The protective role of anti-oxidants in I/R injury is also frequently studied by using this model (Citation4,Citation5).
Cells and tissues naturally possess antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase, which scavenge and remove ROS and repair oxidative damage. Sometimes this natural defense is not enough to handle the overproduction of ROS and consumption of a diet rich in antioxidants has been suggested to contribute to the defense (Citation6). The protective effects of the antioxidants in the diet have been attributed to vitamins C and E, carotenoids, and plant polyphenols such as proanthocyanidins (condensed tannins) and flavonoids (Citation7,Citation8). Rosehips (fruits of roses), which are traditionally used for drinks and jams in several European countries, are a rich source of minerals (K, P), carotenoids (lycopene), vitamins – especially vitamin C (ascorbic acid), folate, and phenolics (Citation7,Citation9–11). Rosehips are thought to be effective in scavenging ROS and preventing lipid peroxidation (Citation12), characteristics ascribed to the high content of antioxidative phenolics (Citation7,Citation9,Citation12). It has been shown previously in the I/R mouse model applied in the present study that a vitamin C concentration equal to that of rosehips failed to protect from lipid peroxidation, which points to the crucial role of antioxidative phenolics (Citation13). Polyphenols are a large group of natural antioxidants and may prevent development of injuries associated with oxidative stress and dependent diseases such as cancer and cardiovascular disease (Citation7,Citation14). Based on structure, which is one or more hydroxyl groups on at least one aromatic ring, phenolics can be divided into different groups such as phenolic acids, flavonoids, stilbenes, and lignans. Flavonoids can be further divided into subclasses, e.g. anthocyanins and flavonols (e.g. catechin) and proanthocyanidins (Citation15). Most of polyphenols are present in food as esters, glycosides, or polymers and cannot be absorbed in their native form (Citation15).
When ingested, some phenolics are hydrolyzed by intestinal enzymes. After hydrolysis to the free agly-cone, polyphenols are detoxicated by conjugation with glycine, glucoronic acid or sulfate in the small intestine and later in the liver. Those conjugated metabolites are transferred to tissues or excreted in urine or bile. The non-absorbed fraction of the polyphenols and the fraction that is excreted back to the small intestine are metabolized by the colonic microbiota (Citation15,Citation16). The microbiota degrades polyphenols into various aromatic acids such as derivates of phenylpropionic, phenylacetic, and benzoic acids with different hydroxylation patterns (Citation14,Citation15). Certain polyphenolic groups, such as proanthocyanidins (condensed tannins), are quite resistant to microbial attack but some bacterial groups are capable of degrading tannins, for example by the possession of tannase (tannin acylhydrolase) (Citation17). Most Lactobacillus species lack tannase but Lactobacillus plantarum, Lactobacillus paraplantarum, and Lactobacillus pentosus, for example, have this enzyme (Citation17).
The aim of the present study was to evaluate the potential of polyphenol active LAB and rosehips from two different rose species with different phenolic profiles, to suppress oxidative stress in the gut. A mouse I/R model was used and the level of lipid peroxidation in the GI tissue was measured as malondialdehyde (MDA). The capability of administered LAB to affect the phenolic profile of proanthocyanidins in the gut content was measured by HPLC-ESI-MS and anthocyanins were analyzed with HPLC-DAD.
Material and methods
Animals
Male Balb/cJ mice (Taconic, 8680 Ry, Denmark), weighing approximately 20 g each were kept under standard laboratory conditions with a controlled 12 h light/dark cycle. Animals were acclimatized 1 week before use and had free access to standard animal chow (R3; Lactamin, Stockholm, Sweden) and tap water. The study was approved in March 2005 by the Ethical Committee for Animal Experimentation (M16-05) at Lund University.
Experimental design
Animals were randomly divided into the following seven groups, with seven mice in each group: I/R control group, with ischemia/reperfusion injury, fed soft standard chow; sham group, without ischemia/ reperfusion injury, fed soft standard chow; RC group, fed standard chow supplemented with Rosa canina (RC); RC + LAB group, fed standard chow supplemented with R. canina and a mixture of eight different polyphenol active Lactobacillus strains (LAB); RP group, fed standard chow supplemented with Rosa pimpinellifolia (RP); RP+LAB group, fed standard chow supplemented with R. pimpinellifolia and a mixture of eight different polyphenol active LAB; LAB group, fed standard chow supplemented with a mixture of eight different polyphenol active LAB.
Each animal was placed in its own cage with a food dish. After 7 days of acclimatization, animals were fed experimental diets for 10 days. The daily dose of each rosehip species was 1.6 g/mouse. To provide each animal with the same amount of energy, the I/R control group, sham group, and LAB group were given 8.7 g of standard chow per mouse per day while rosehip groups were given 7.4 g chow per mouse per day. The animal chow R3 was dissolved in water to achieve a softer consistency before the addition of the different supplements. Rosehips used in the experimental diets originated from two species of roses grown at Balsgård (Sweden), i.e. Rosa pimp-inellifolia (RP) and Rosa canina (RC). The rosehips had been freeze-dried and ground up. LAB consisted of the strains Lactobacillus plantarum 299v, L. plan-tarum HEAL19, L. plantarum RESO49, L. plantarum RESO56, Lactobacillus paraplantarum RESO97, Lactobacillus pentosus LP2, L. pentosus 9T, and L. plantarum subsp. argentoratensis (CCUG 50787T); all except the last strain are known to be of human origin. The strains were mixed in a freezing medium (0.85 g/l dipotassium hydrogen phosphate, 0.2 g/l potassium dihydrogen phosphate, 0.6 g/l trisodium citrate dihydrate, 0.25 g/l magnesium sulfate heptahydrate, 121 ml 99.5% glycerol, 879 ml distilled water) with 108 cfu/ml of each strain. Dose per day and cage was approximately 8×108 cfu.
Intestinal ischemia-reperfusion and sampling
Anesthesia comprising 7.5 mg ketamine (Ketalar 50 mg/ml; Pfizer, UK) and 2.5 mg xylazine (Narcoxyl 20 mg/ml; Veterinaria AG, Schweiz) per 100 g body weight was injected intraperitoneally into the mice, and the animal was placed on a 37°C warming pad. A midline abdominal incision was made, then the superior mesenteric artery was identified and occluded with a vessel clamp for 30 min to obtain ischemia of the small intestine and colon. The peritoneal cavity was filled with 1 ml Dulbecco's phosphate-buffered saline (PBS) for fluid resuscitation. After 30 min, the clamp was removed, which resulted in immediate reperfusion. The abdomen was closed using a running Vicryl 4-0 suture (Johnson & Johnson, USA). The animal was placed back into the cage and allowed to awake from anesthesia. After 240 min, the animal was anesthetized again, sacrificed, and sampled. The sham group was subjected to the surgical procedure described above but without clamping of the superior mesenteric artery. Cecum stool was used for polyphenol analyses and rinsed cecum tissue for determination of lipid peroxidation (MDA). The part of the ascending colon tissue used for viable counts was placed in 3 ml of freezing medium and remaining pieces were rinsed in ice-cold Dulbecco's PBS and used for determination of MDA. All samples were weighed, frozen in liquid nitrogen (except for microflora sample), and stored at −70°C until analysis. Surgery was performed with attention to sterile technique.
Viable count
The colonic tissue samples were stored in 3 ml of freezing medium (described earlier) at −80°C until the time for analysis. Samples were sonicated for 5 min and vortexed for 2 min. One ml of the sample was mixed with 9 ml of dilution liquid (8.5 g/l sodium chloride, 1 g/l bacteriological peptone, 1 g/l Tween 80, and 0.2 g/l L-cysteine monochloride monohydrate) and serially diluted. After dilution, 0.1 ml of the samples from appropriate dilutions were spread with glass beads (5 mm) on Rogosa agar (Oxoid Ltd, Basingstoke, Hampshire, UK) plates that were incubated anaerobically (Gas Pack System, Gas Pack, Becton Dickinson Microbiology Systems, Cock-eysville, MD, USA) for 72 h at 37°C (lactobacilli count) and violet red-bile-glucose agar plates (VRBG; Merck KgaA, 64271 Darmstadt, Germany) that were incubated aerobically for 24 h at 37°C (Enterobacteriaceae count).
Randomly amplified polymorphic DNA (RAPD)
Four to 12 colonies with a morphology typical of the administered strains were randomly picked from the countable Rogosa plate. All strains in the LAB mixture had the same colony morphology, typical of L. plantarum. Isolates were recultured on Rogosa agar to secure purity. The pure cultures were washed twice in sterile Milli-Q water and resuspended in 250 μl of sterile Milli-Q water. The cells were disrupted and DNA was recovered by shaking with glass beads (2 mm) for 45 min at 4°C. Pellets were obtained by centrifugation at 20 817 g for 5 min. The supernatant was used for the PCR reaction.
PCR amplification and RAPD typing were in principle performed as described by Quednau et al. (Citation18): 1 μl of the supernatant was mixed with 49 μl of master mixture containing 10× PCR reaction buffer with 1.5 mM MgCl2 (Roche Diagnostics GmbH, Mannheim, Germany), 10 mmol of each nucleotide (Roche), 5 U/μl Taq DNA polymerase (Roche), 9-mer primer with sequence 5-ACG CGC CCT-3’ (Scandinavian Gene Synthesis AB, Köping, Sweden), and sterile Milli-Q water. The reaction mixture was cycled according to the following temperature profile: 94°C for 45 s, 30°C for 120 s, 72°C for 60 s for four cycles followed by 94°C for 5 s, 36°C for 30 s (with extension of 1 s for each cycle), 72°C for 30 s for 26 cycles. The PCR reaction was terminated at 72°C for 10 min and thereafter cooled to 4°C. The PCR products were visualized by agarose gel electrophoresis. A DNA molecular weight marker VI (Roche) was used as standard. Bands were visualized at 302 nm with a UV transilluminator and photographed. Images were analyzed by BioNumerics 2.5 (Applied Maths, Kortrijk, Belgium) using the Pearson product moment correlation coefficient (r) and UPGMA (unweighted pair group method with arithmetic averages).
Malondialdehyde (MDA)
Malondialdehyde (MDA) is used as an indicator of lipid peroxidation. MDA-586TM (Oxis International Inc., Portland, OR, USA), a colorimetric assay, was used to determine MDA in collected and PBS-washed segments of colon and cecum tissues that had been stored at −70°C. Tissue samples were homogenized in 1 ml cold Dulbecco's PBS and 10 μl butylated hydroxytoluene (5 mM). After homogenization samples were centrifuged at 4000 g for 10 min at 4°C. Lipid peroxidation was estimated by mixing 200 μl of the supernatant with 10 μl probucol, 640 μl of diluted N-methyl-2-phenylindol, and 150 μl concentrated hydrochloric acid (12 M). The samples were then incubated in a water bath at 45°C for 60 min and centrifuged at 10 000 g for 10 min at 4°C. The absorbance of the supernatant was measured by spectrophotometry at 586 nm. Since MDA is not stable, tetramethoxy-propane (TMOP) was used as an MDA standard. The TMOP is hydrolyzed and generates MDA during the acid incubation step at 45°C. MDA was expressed as nmol/g tissue.
Extraction
Cecum content was collected, weighed, frozen in liquid nitrogen, and stored at −70°C. For extraction, each sample was divided into two tubes by weight and 0.75 ml of 1% TFA (trifluoroacetic acid, 99%, Aldrich) in methanol (Lichrosolv for liquid chromatography; Merck) was added. Samples were vortexed and placed in an ultrasonic bath for 5 min. Thereafter firm parts of the samples were crushed with a spoon and samples were vortexed and put in the ultrasonic bath for another 5 min. After centrifugation for 10 min supernatants were evaporated under nitrogen gas and dissolved in 120 μl of 1% TFA in methanol solution.
Total antioxidant capacity – FRAP assay
The ferric reducing ability of plasma (FRAP) assay was performed as described previously by Benzie and Strain (Citation19). Briefly, FRAP reagent was prepared by mixing 25 ml of 300 mmol/l acetate buffer, pH 3.6 (3.1 g C2H3NaO2*3H2O (Riedel-de Haen, Germany) and 16 ml C2H4O2 (BDH Laboratory Supplies, UK) per liter buffer solution) with 2.5 ml of 10 mmol/l TPTZ (2,4,6-tripyridyl-s-triazine (Fluka Chemicals, Switzerland) in 40 mmol/l HCl (BDH)) and 2.5 ml of 20 mmol/l FeCl3*6H2O solution (BDH). FRAP reagent was heated to 37°C before use. Samples were extracted as described previously and diluted with 90% methanol. Antioxidative capacity was read by adding 10 μl of sample to 1 ml of reagent. A UV spectrophotometer (Shimadzu UV-2101 PC) was used for recording the absorbance in the samples at 593 nm with a monitoring period of every 10 s for a total of 240 s. A standard curve was made by using FeSO4*7H2O (Fe2+) (Fluka) at concentrations 200, 400, 800, and 2000 μmol/l. FRAP values were expressed as mmol/100 g ww (wet weight).
Total phenolic content measured by Folin-Ciocalteau reagent
Each reaction tube contained 10 μl of sample, 100 μl of 5% ethanol, 200 μl of Folin-Ciocalteau reagent, 2 ml of 15% Na2CO3, and 1 ml of milli-Q water, and was allowed to react for 2 h before reading of absorbance at 765 nm. Gallic acid was used as standard solution; 12 mg gallic acid was dissolved in 5 ml concentrated ethanol and milli-Q water was added up to 100 ml volume. A standard curve was made with concentrations of 5, 10, 20, 50, and 100 μg/ml by addition of 5% ethanol. Total phenol content was expressed as mg/g ww.
Analysis of phenolic compounds with HPLC-ESI-MS
Proanthocyanidins. Analysis was performed using a Perkin Elmer Series 200 LC system equipped with a UV detector set at 280 nm and connected to a Perkin Elmer PE SCIEX API 150 single quadrupole mass spectrometer with a turbo ionspray interface; 7 μl of sample was injected onto the guard column Lichrospher 100RP-18 (5 μm, Merck). Separation was accomplished on a Superspher 100 RP-18 column (75×4 mm i.d., 4 μm, Merck) and eluated at 1 ml/min using 0.4% formic acid (A) and acetonitrile (B) as mobile phase. The gradient program was adjusted as described by Salminen et al. (Citation8): 0–3 min, 100% A; 3–30 min, 100–70% A and 0–30% B; 30–35 min, 70–60% A and 30–40% B; 35–38 min, 60% A and 40% B; 38–42.5 min 100% A. The spectrometer was operated in the negative mode with a split ratio of 1:4. Spray needle voltage, orifice plate voltage, and ring voltage were set at −4000 V, −35 V, and −220 V, respectively. Heated nitrogen gas temperature was set at 35°C. Nebulizer gas was set at 9 and curtain gas at 12. Mass spectra were obtained by scanning between 125 and 1200 amu and by extracting selected ions and fragments as described by Salminen et al. (Citation8). Standards were as follows: (+)-catechine hydrate (Sigma), 4-hydroxybenzoic acid (Fluka), protocatechuic acid (Sigma), vanillic acid (Fluka), syringic acid (Sigma).
Anthocyanins. Extracted samples were diluted four times in 1% TFA in methanol before chromatographic analysis of anthocyanins. A Shimadzu instrument was used, consisting of LC-10ADVP gradient pump, system control SCL-10AVP, and diode-array detector SPD-M10AVP. A guard column Lichrospher 100 RP-18 (5 μm, Merck) was installed before the analytical column. Sample (15 μl) was separated on the Superspher 100 RP-18 column (75×4 mm i.d., 4 μm, Merck) and eluated at 0.8 ml/min using as mobile phase 10% formic acid (A) and acetonitrile (B). All chromatographic procedures were perfor med at room temperature. The wavelength used for detection was 510 nm. Cyanidin-3-glycoside (Extra Synthés, France) was used as standard. Samples and standard were plotted and quantified on the basis of peak area. The gradient program was performed according to Nyman et al. (Citation20) with some changes: 0.01 min, 10% acetonitrile; 3 min, 15% acetonitrile; 12 min, 25% acetonitrile; 14 min, 50% acetonitrile; 17 min, 10% acetonitrile.
Statistics
All statistical analyses were performed with Sigma Stat 3.1 (SPSS Inc.). Since data were not normally distributed, ANOVA on ranks was the method of choice to test for significant treatment effects. Differences between all groups were evaluated by the Kruskal–Wallis test, while the Mann–Whitney rank sum test was used to evaluate differences between two groups. Results were considered statistically significant when p<0.05. Values are presented as median (25th–75th percentiles).
Results
Intestinal I/R
All animals survived the I/R procedure, and reperfusion was performed and observed in all the animals. Colonic bleeding was observed in one animal in the RP group during the operation and this animal was excluded from statistical evaluations.
Viable count
The lactobacilli count in colonic tissue varied between 1×105 and 2.6×106 cfu/g, and no significant differences were found between groups. The Enterobacteriaceae count varied between <102 cfu/g and 103 cfu/g, and no significant differences could be seen between groups.
Isolation of administered strains in the gut
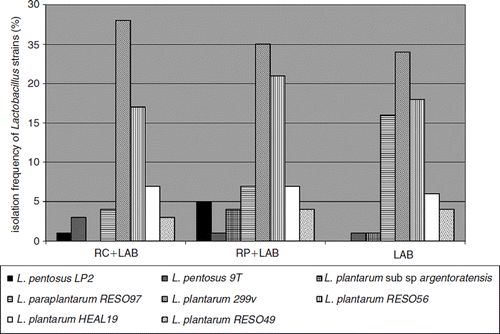
The different administered Lactobacillus strains were only found in animals given LAB (LAB, RP + LAB, and RC + LAB groups). All administered strains could be re-isolated from the colonic tissue. The most frequently occurring strain was L. plantarum 299v, followed by L. plantarum RESO56, L. paraplantarum RESO97, and L. plantarum HEAL19 (). A difference between the group given LAB alone compared with the two where LAB was given together with rosehips, was that L. paraplantarum RESO97 was re-isolated to a higher degree in the LAB group, while no L. pentosus LP2 was isolated from this group ().
Figure 1. Isolation frequency from cecum content of the different Lactobacillus strains administered to mice with dietary supplements of rosehips and a mixture of polyphenol active Lactobacillus strains (LAB). RC+LAB was given standard diet supplemented with rosehips of R. canina and LAB; RP+LAB, standard diet supplemented with rosehips of R. pimpinellifolia and LAB; LAB group was given standard diet supplemented with LAB.
Malondialdehyde (MDA)
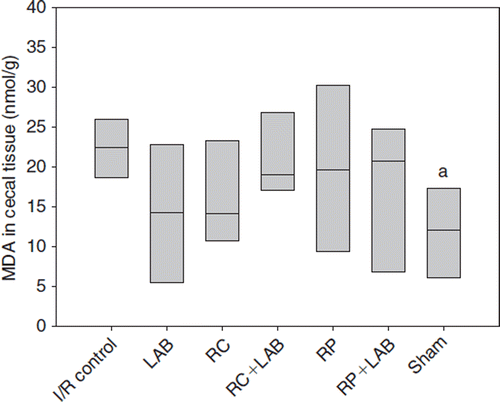
MDA was significantly increased in the cecum tissue of the I/R control group compared with the sham group (p=0.026) (). However, none of the dietary supplements significantly lowered the MDA level, even if the median values of all treatment groups were lower than for the I/R control, a trend that was most pronounced for the LAB and RC groups ().
Figure 2. Malondialdehyde (MDA) in cecal tissue after ischemia and reperfusion (I/R) of mice administered different diets supplemented rosehips and polyphenol active Lactobacillus strains (LAB): I/R control was given standard diet; LAB, standard diet supplemented with LAB; RC, standard diet and rosehips of R. canina; RC+LAB, standard diet, rosehips of R. canina, and LAB; RP, standard diet and rosehips of R. pimpinellifolia; RP+LAB, standard diet with rosehips of R. pimpinellifolia and LAB; sham group was given standard diet. adenotes p<0.05 compared to I/R control.
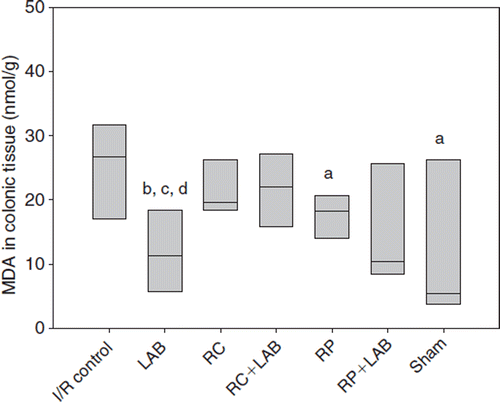
In the colon tissue, MDA was significantly higher in the I/R control group than in the sham group (p=0.038), RP group (p=0.035), and LAB group (p=0.007) (). The LAB group also showed a significantly lower MDA value than the RC group (p=0.017) and RC+LAB group (p=0.011). The median value of the RP+LAB group is strikingly low compared with the I/R control but no statistical significance was reached (p=0.097 compared with I/R control; ).
Figure 3. Malondialdehyde (MDA) in colonic tissue after ischemia and reperfusion (I/R) or mice administrated different diets supplemented with rosehips and polyphenol active Lactobacillus strains (LAB): I/R control was given standard diet; LAB, standard diet supplemented with LAB; RC, standard diet and rosehips of R. canina; RC+LAB, standard diet, rosehips of R. canina, and LAB; RP, standard diet and rosehips of R. pimpinellifolia; RP+LAB, standard diet with rosehips of R. pimpinellifolia and LAB; sham group was given standard diet.a denotes p<0.05 compared to control; bdenotes p<0.01 compared to control; cdenotes p<0.05 compared to RC group; ddenotes p<0.05 compared to RC+LAB group.
Total antioxidant capacity (FRAP)
Total antioxidant capacity measured as FRAP value in cecal content was significantly higher in all groups with a dietary supplement of rosehips (RC, RC + LAB, RP, and RP+LAB groups) compared with the I/R control, sham, and LAB groups (). No significant differences were reached between the different rosehip groups, even if there was a trend that a combination of bacteria and rosehips increased the FRAP values in the cecal content ().
Table I. Total antioxidant capacity (FRAP) in cecal content of mice administered different dietary supplements of rosehips and a mixture of polyphenol active Lactobacillus strains (LAB).
Total phenolic content measured with Folin-Ciocalteau reagent
Total phenolic content measured with Folin-Ciocalteau reagent was significantly higher in all groups given dietary rosehip supplements (RC, RC + LAB, RP, and RP+LAB groups) compared with the I/R control, sham, and LAB groups (). No significant differences were reached between the different groups administered rosehips with or without lactobacilli. However, there is a trend in the medians that the combination of rosehips and lactobacilli yielded higher values than rosehips alone ().
Table II. Total phenolic content measured with Folin-Ciocalteau reagent in cecal content of mice administered different dietary supplements of rosehips and a mixture of polyphenol active Lactobacillus strains (LAB).
Proanthocyanidins in cecum
Proanthocyanidins analyzed in cecum content of mice administered different dietary supplements were: catechin (HPLC peak corresponding to m/z 289 rt= 13.9 min), metabolite I (m/z 291 rt= 16.04 min), proanthocyanidin-monomer-glycoside (m/z 451 rt=12.6 min), proanthocyanidin-dimer-monoglycoside (m/z 739.3 rt= 12.62 min), proanthocyanidin-dimer-diglycoside (901.3 rt=12.7 min), 3,4-dihydroxybenzoic acid (protocatechuic acid; m/z 153 rt=8.5 min). All analyzed phenolics increased in animals given rosehips while no significant differences were found between the I/R control, LAB, and sham groups (). A general trend, and in some cases supported by statistically significant differences (), was that catechin and metabolite I were higher in rosehips of R. canina than in R. pimpinellifolia, and that LAB supplementation of the rosehips increased the concentrations (). The same trend was true for proanthocyanidin-monomer-glycoside ().
Table III. HPLC-ESI-MS analysis of proanthocyanidins in cecal content of mice administered different dietary supplements of rosehips and a mixture of polyphenol active Lactobacillus strains (LAB).
Proanthocyanidin-dimer-monoglycoside and the corresponding diglycoside were found in significantly higher concentrations in groups given R. canina compared with R. pimpinellifolia ().
3,4-Dihydroxybenzoic acid (protocatechuic acid, PCA) was significantly higher in the cecum content of RP and RP + LAB groups compared with all other groups (p<0.001) ().
Anthocyanins in cecum
Cyanidin-3-O-glycoside was detected in all groups supplemented with rosehips. Concentrations were significantly higher (p<0.001) in the RP group (17.21 (8.4–21.94)) than in the RC group (0.56 (0.4–0.62)). Also, the RP+LAB group (19.59 (16.19–25.46)) had a significantly higher content of anthocyanin (p<0.001) than the RC+LAB group (0.53 (0.46–0.66)). The concentration of cyanidin-3-O-glycoside in the RP+LAB group was higher than in the RP group, but the difference was not statistically significant.
Discussion
Reperfusion of ischemic tissue results in the formation of toxic ROS, that directly damage cellular membranes by lipid peroxidation and stimulate activation of leukocytes (Citation6). MDA is a by-product of lipid peroxidation induced by free radicals and is used as an indicator of oxidative stress (Citation21). MDA values increase in I/R (Citation5). This was true also in the present study, where the MDA levels in both cecal and colonic tissue were significantly lower in the sham group than in the I/R control ( and ). All groups receiving dietary supplementation showed lower median values of MDA than the control. Rosehips of R. pimpinellifolia, with and without LAB decreased MDA levels in colon more efficiently than rosehips of R. canina (). According to the HPLC-MS analyses there is a difference in the polyphenolic profile between the two rose species. Rosehips of R. canina seem to have a higher content of proanthocyanidins (PA) while rosehips of R. pimpinellifolia are richer in anthocyanins. A majority of the polymeric PA and anthocyanins are not directly absorbed from the small intestine, but are degraded into low-molecular-weight compounds by the colonic microbiota in cecum and colon (Citation22). MDA reduction by rosehips may be due to the accumulation of intact polyphenols, but maybe also by their degradation products in cecum and colon. It can be speculated that several of the break-down products of the larger polyphenols can possess higher anti-oxidative properties than the native compounds and more efficiently prevent lipid peroxidation. This is supported by the trends shown in the FRAP analyses ().
It has previously been shown in the I/R model used in the present study that a dietary supplement of rosehips (R. canina) and the single strain, Lactobacillus plan-tarum DSM 9843 (= strain 299v), could reduce cecal tissue MDA levels. Furthermore, increased MDA correlated with increased viable count of Enterobacteriaceae (Citation13). A similar correlation was shown also by Xing et al. (Citation23) in an I/R liver injury model in rats, where the objective was to clarify the intestinal status after an I/R injury in the liver. The results showed elevated MDA levels in the liver tissue as well as increased intestinal Enterobacteriaceae count and bacterial translocation. In the present study, the Enterobacteriaceae counts were low in all groups, and presumably they were low from the start in this particular batch of mice. This can be one factor that explains the differing results between the present study and that by Hå;kansson et al. (Citation13). However, another explanation can be that instead of using just one Lactobacillus strain (L. plantarum 299v) (Citation13), a mixture of eight different strains were used in the present study, i.e. L. plantarum 299v and seven other strains. Different strains possess different characteristics, they can interact with each other and with different components of the resident microbiota.
The lowest MDA values in the present study were observed in the LAB group fed eight different Lactobacillus strains. All administered strains, except for L. pentosus LP2, were found in high numbers in the cecal content. Lactic acid bacteria as such can have antioxidative activity (Citation24,Citation25). Certain strains of L. acidophilus, L. bulgaricus, and L. fermentum are able to chelate metal ions, scavenge ROS, and possess reducing activity (Citation24,Citation25). Also it has been shown that six strains of Lactobacillus acidophilus and two strains of Bifidobacterium longum were able to scavenge MDA (Citation25). Even if our strains were not tested separately for their antioxidative abilities, at least one of them must possess this characteristic and scavenge ROS after reperfusion and decrease MDA. In the present study LAB alone significantly decreased MDA levels in colon compared with the control and R. canina groups. It was noticed that L. paraplantarum RESO97 was isolated in higher frequency from the LAB group than from groups where LAB was combined with rosehips. Evidently, L. paraplantarum RESO97 possess antioxidative activity and scavenging abilities towards ROS and MDA to a higher degree than the other LAB strains.
Antioxidative capacity was measured using the FRAP assay, while total phenolics were determined with Folin-Ciocalteau reagent. The FRAP assay is a direct measurement of antioxidant as reductants in a redox-linked colorimetric reaction (Citation19). Groups fed rosehips in combination with LAB showed increased antioxidant capacity as well as higher total phenolics values compared with the groups that received rosehips and LAB alone. Antioxidant capacity in the cecal content is supposed to be correlated with total phenolics (Citation7).
The administered LAB appear to have a higher metabolic activity towards the polyphenols in rosehips than the resident microbiota of the mouse. The administered strains were all positive when tested for tannase activity (data not shown). They probably use tannase to break down the polyphenols with a high degree of polymerization into the simpler compounds such as phenolic acids, PA monomers, dimers, and trimers. These aromatic acids and low-molecular-weight compounds might protect against oxidative stress by having reducing and antioxidative properties (Citation14) and probably contribute to an increase in the total antioxidant activity and total phenolics. Gao et al. (Citation7) showed that phenolic extracts made up the major contribution to the antioxidative activity of the rosehips while ascorbic acid and lipophilic extract contributed to a much lesser extent. Furthermore, it has been shown in the I/R model that pure vitamin C in the concentration valid for rosehips did not have any effects on MDA (Citation13).
Proanthocyanidins (PA), or condensed tannins, consist of different flavan-3-ol subunits, also called proanthocyanidin monomers, or catechins. These units are linked mainly through C4→C8 bond to form oligomers and polymers, of which several are glycosylated (Citation6). In the present study catechin, metabolite I, and some PA monomer- and dimer-glycosides were examined (). It was observed that the concentration of the analyzed compounds had a tendency to increase when rosehips were given in combination with LAB, which suggests that LAB were able to degrade polymeric PA and produce compounds of lower molecular weight. PA can be quite resistant to microbial attack. The fact that LAB were able to produce catechin and PA dimers is favorable, since it has been suggested that absorption of PA depends on the degree of polymerization. PA degradation would start with depolymerization of oligomers and polymers into trimers, dimers, catechin monomers, and finally phenolic acids. These monomers, dimers, and phenolic acids might be absorbed and reach the systemic circulation (Citation6,Citation26) where they are believed to act as antioxidants and contribute to the protection of tissues against oxidative stress (Citation14,Citation26).
Anthocyanins are secondary metabolites of plants and are part of the flavonoid family. They are responsible for the red, purple, and blue colors of fruits, vegetables, and flowers. Anthocyanins might possess antioxidative, antimicrobial, anti-inflammatory, and anticarcinogenic properties (Citation22). The HPLC-DAD analysis of anthocyanins in cecal content of mice fed R. canina and R. pimpinellifolia, respectively, showed one peak identified as cyanidin-3-O-glycoside. Previous analyses of phenolic compounds in R. canina (Citation9) and in black rosehips (Citation27) confirm the results. In the present study cyanidin-3-O-glycoside was found only in small amounts in R. canina compared with R. pimp-inellifolia, which contained significantly higher amounts of the anthocyanin. This could be expected due to the dark, blue color of R. pimpinellifolia. It is well documented that most of the administered anthocyanins reach the end of the small intestine in the intact form and continue further to the colon where they are metabolized by the colonic microbiota to aglycones, and then to phenolic acids (Citation22,Citation28). The free aglycones, called anthocyanidins, are rarely found in dietary plant materials because of their instability (Citation22). According to different metabolic studies (Citation22) cyanidin-3-O-glycoside (cy-3-gly) is converted to its unstable aglycone by cleavage of the sugar moiety, cyanidin (cy), which is further degraded by the microbiota to protocatechuic acid (PCA). Interestingly, the RP+LAB group showed higher concentrations of cy-3-gly than the group without LAB (RP). Thus, the resident microbiota seems to have a higher metabolic activity towards cy-3-gly than LAB. LAB appears to somehow slow down the deglycosylation and degradation of the anthocyanin. The result for the protocatechuic acid was the opposite. PCA concentration was higher in the group fed RP than that fed RP+LAB. If the PCA is a metabolite of cy-3-gly degradation, this result correlates with the observed anthocyanin concentrations, i.e. when concentration of cy-3-gly was high the concentration of PCA was low and vice versa. PCA is a benzoic acid derivate. There are contradictory data in the literature on the biological activity of protocatechuic acid. It is believed to possess antioxidant and free radical scavenging, anticarcinogenic, and antibacterial activities, but it also exhibited prooxidant activities and induced oxidative stress (Citation29). Effects of PCA on oxidative stress seem to be dose and time dependent (Citation30). At a low dosage, PCA exerted a protective effect on oxidative stress, while at elevated dosages it induced oxidative stress (Citation29,Citation30). In light of the results of the present study, it seems more preferable to combine LAB and RP than to use RP alone, due to the slower degradation of cy-3-gly to protocatechuic acid in presence of LAB. The protocatechuic acid is produced at lower dosage and can be absorbed faster from the gut. The group fed RP+LAB showed the lowest MDA level (median value) in the colonic tissue, which might confirm the results ().
In conclusion, the present study demonstrates that administration of rosehips in combination with polyphenol active LAB decreased MDA levels in colonic tissue and, hence, prevented lipid peroxidation and oxidative stress after intestinal I/R injury. Furthermore, when LAB was given together with rosehips, highly polymerized proanthocyanidins were presumably degraded, which resulted in increased concentration of more readily absorbed, antioxidative catechin, catechin eqvivalents, and PA dimers. The antioxidative capacity and total phenols in cecal content were also increased. The LAB seem to slow down degradation of cyanidin-3-O-glyco-side in R. pimpinellifolia and its conversion to protocatechuic acid and in that case may prevent oxidative stress. According to our results, there is no strong evidence yet but an indication that rosehips, especially R. pimpinellifolia, together with polyphenol active probiotics, may prevent and suppress I/R injury in the intestines.
Acknowledgments
The project was financed by VINNOVA, the Swedish Governmental Agency for Innovation Systems. Jonas Björk, biostatistician, Competence Center for Clinical Research, Lund University Hospital is gratefully acknowledged for the statistical analyses. All authors participated in the study design, evaluation of the results, and finalization of the manuscript. There were no conflicts of interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine” held in Paris, June 14, 2002. Am J Clin Nutr 2003;78:675–83.
- Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol 1995;3:149–54.
- Grisham MB, Granger DN. Neutrophil-mediated mucosal injury – role of reactive oxygen metabolites. Dig Dis Sci 1988; 33:6S–15S
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001;94:1133–8.
- Thomson A, Hemphill D, Jeejeebhoy KN. Oxidative stress and antioxidants in intestinal disease. Dig Dis 1998;16:152–8.
- Rasmussen SE, Fredriksen H, Krogholm KS, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bio-availability, and protection against cardiovascular disease. Mol Nutr Food Res 2005;49:159–74.
- Gao X, Björk L, Trajkovsli V, Uggla M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J Sci Food Agric 2000;80:2021–7.
- Salminen J-P, Karonen M, Lempa K, Liimatainen J, Sinkkonen J, Lukkarinen M, . Characterisation of proanthocyanidin aglycones and glycosides from rosehips by high-performance liquid chromatography-mass spectrometry, and their rapid quantification together with Vitamin C. J Chromatogr A 2005;1077:170–80.
- Hvattum E. Determination of phenolic compounds in rosehip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun Mass Spectrom 2002;16:655–62.
- Böhm V, Fröhlich K, Bitsch R. Rosehip – a new source of lycopene? Mol Aspects Med 2003;24:385–9.
- Strålsjö L, Alklint C, Olsson EM, Sjöholm I. Total folate content and retention in rosehips (Rosa spp.) after drying. J Agric Food Chem 2003;51:4291–5.
- Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T, . Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res 2002;16:157–61.
- Håkansson Å, Stene C, Mihaescu A, Molin G, Ahrné S, Thorlacius H, . Rosehip and Lactobacillus plantarum DSM 9843 reduce ischemia-reperfusion injury in the mouse colon. Dig Dis Sci 2006;51:2094–101.
- Gonthier M-P, Cheynier V, Donovan LJ, Manach C, Morand C, Mila I, . Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr 2003;133:461–7.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47.
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130:2073–85.
- Osawa R, Kuroiso K, Goto S, Shimizu A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl Environ Microbiol 2000;66:3093–7.
- Quednau M, Ahrné S, Petersson AC, Molin G. Identification of clinically important species of Enterococcus within 1 day with Randomly Amplified Polymorphic DNA (RAPD). Curr Microbiol 1998;36:332–6.
- Benzie FFI, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6.
- Nyman NA, Kumpulainen TJ. Determination of anthocyanidins in berries and red wine by High-Performance Liquid Chromatography. J Agric Food Chem 2001;49:4183–7.
- Shi G-F, An L-J, Jiang B, Guan S, Bao Y-M.Alphinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci Lett 2006;403:206–10.
- Keppler K, Humpf H-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem 2005;13:5195–205.
- Xing H, Li L, Xu K, Shen T, Chen Y, Sheng J, . Intestinal microflora in rats with ischemia/reperfusion liver injury. J Zheijang Univ Sci 2005;6B:14–21.
- Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, . Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 2002;72:215–24.
- Lin M-Y, Yen C-L. Inhibition of lipid peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J Agric Food Chem 1999;47:3661–4.
- Gonthier M-P, Donovan LJ, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med 2003;35:837–44.
- Oszmianski J, Kalisz B, Kalisz S. Influence of skullcap flavones on colour, anthocyanin stability and antioxidant activity of some berry juices. Sci Res 2001;12:496–500.
- Kahle K, Kraus M, Scheppach W, Ackermann M, Ridder F, Richling E. Studies on apple and blueberry fruit constituents: do the polyphenols reach the colon after ingestion? Mol Nutr Food Res 2006;50:418–23.
- Babich H, Sedletcaia A, Kenigsberg B. In vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: involvment in oxidative stress. Pharmacol Toxicol 2002;91:245–53.
- Nakamura Y, Torikai K, Ohto Y, Murakami A, Tanaka T, Ohigashi H. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose- and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis 2000;21:1899–907.