Abstract
Objective: Our previous study suggested that phenols (phenol and p-cresol) produced by gut bacteria affect the skin in hairless mice. In the present study we aimed to determine if the same phenomenon is applicable to humans. Methods: First, we analyzed the correlation between serum phenol levels and corneocyte size in 50 healthy female volunteers. Second, we administered a prebiotic beverage (containing galacto-oligosaccharides and polydextrose) to 19 healthy female volunteers and examined the correlations among fluctuation of phenol levels, corneocyte size, and cathepsin L-like activity. Finally, we performed an in vitro experiment using monolayer-cultured human keratinocytes to determine whether phenols physiologically affected keratinocyte differentiation. Results: In 50 healthy female volunteers, serum phenol levels showed a significant inverse correlation with skin corneocyte size. When a prebiotic beverage was administered for 3 weeks to 19 healthy female volunteers, p-cresol levels decreased significantly, and significant increases in corneocyte size and cathepsin L-like protease activity were seen. On in vitro examination, monolayer-cultured human keratinocytes subjected to 20 nmol/ml phenol or p-cresol failed to express K10 protein (a molecular marker for normal keratinocyte differentiation) under physiological conditions. Conclusions: These results suggest that phenols produced by gut bacteria adversely affect keratinocyte differentiation in female human skin. Furthermore, these results indicate that serum phenol levels are appropriate biomarkers of keratinocyte differentiation disturbances caused by an undesirable intestinal environment in humans.
Introduction
There is a popular opinion that the intestinal environment is closely related to the condition of the skin in Japanese women. Similarly, it is often said that persistent constipation is correlated with skin problems. A previous report suggested that skin disorders are caused by metabolites produced by gut bacteria (Citation1), but details have not been provided.
Phenols (phenol and p-cresol) are metabolites of aromatic amino acids produced by gut bacteria in animals (Citation2). A previous study (Citation3) showed that specific bacteria, including Proteus sp., Streptococcus faecalis Bacteroides fragilis Fusobacterium sp., and Clostridium sp., produced phenols under unfavorable gut conditions (such as alkalinity), so phenols are often used as biomarkers of the intestinal environment (Citation4).
Although a certain amount of phenols is likely to remain unabsorbed and be excreted in the feces, an excess of phenols, caused by any of several factors - including a protein-rich diet, could be absorbed by the gut and may cause undesirable effects in the body (2,5).
In our previous study (Citation6) in hairless mice, including gnotobiotic experimentation, we confirmed that phenols produced by cecum bacteria are absorbed and distributed by the blood, and eventually accumulate in the skin. At that stage, a yellowish dullness, detected by the color exam meter's b* value, and incomplete cornification, exhibited by decreased corneocyte size, were observed. These results indicate that phenols produced by gut bacteria cause a skin problem in mice.
In this study, we aimed to substantiate that the above phenomenon applies to humans. We analyzed the correlation between serum phenol levels and parameters of skin condition in healthy female volunteers. We also performed an in vitro experiment using monolayer-cultured human keratinocytes to determine whether phenols physiologically affect keratinocyte differentiation.
Our findings suggest that phenols produced by gut bacteria adversely affect keratinocyte differentiation in female human skin.
Material and methods
Volunteers
Fifty healthy female volunteers participated in a correlation analysis between serum phenol levels and corneocyte size. The mean age of participants was 39.1 years (range 21–60 years); 14 subjects were in their 20s, 14 were in their 30s, 11 were in their 40s, and 11 were in their 50s–60s. Nineteen healthy female volunteers participated in a prebiotic beverage administration trial. Their mean age was 31.9 years (range 21–39 years).
All participants were free of medical treatment for 1 month before and during the experiment. They also were free of severe bowel movement trouble, and their defecation frequency exceeded four times per week. Because we could not find any lifestyle parameter, including age, diet, weight, sleeping hours, exercise, menstrual status, or birth experience, that significantly correlated with serum phenol levels or corneocyte size (data not shown), we excluded these parameters from further analysis.
Trial for correlation analysis between serum phenol levels and corneocyte size
After eating an identical lunch menu, the 50 volunteers were taken to an air- and humidity-controlled room. Then, corneocytes from their inner forearm stratum corneum were collected by tape stripping to measure corneocyte size (described below), and their blood was collected from an arm vein to measure serum phenol levels (described below). Both collections from all volunteers were performed within 3 h of the lunch.
Administration trial
Our questionnaire results indicate that the 19 volunteers refrained from taking any antibacterial agents and probiotic or prebiotic foods, except the test products, for the 3 weeks before and during the trial. A prebiotic beverage (HiLine®; Yakult Honsha Co., Ltd, Tokyo, Japan) containing 3.0 g galacto-oligosaccharides and 5.6 g polydextrose () per day was administered for 21 days.
Table I. Nutritional data for the prebiotic beverage.
On day 0 and day 22, corneocytes and blood were collected as in the trial of the 50 volunteers described above. Then, corneocyte size, cathepsin L-like protease activity level in the corneocytes, and serum phenol levels on day 0 (before administration) and day 22 (after administration) were measured (described below).
Analyses of stratum corneum characteristics
In the trials with the 50 and the 19 volunteers, second layer corneocytes of the inner forearm stratum corneum were gathered by tape stripping (Skin Checker; Promotool Corp., Tokyo, Japan) to measure corneocyte size (an indicator of keratinocyte terminal differentiation condition). The tape-attached corneocytes were stained with 0.5% (w/v) Brillant Green (Merck Japan, Tokyo, Japan) and 1.0% (w/v) Gentian Violet B (Kanto Chemical, Tokyo, Japan). The corneocytes were observed and photographed using a digital microscope (Hi-Scope KH-3000; HiRox, Tokyo, Japan), and the corneocyte size was measured using an image analyzer (Win Roof; Mitani Co. Ltd, Fukui, Japan). The average size of 30 corneocytes per volunteer was calculated.
In the trial with the 19 volunteers, third layer corneocytes of the inner forearm stratum corneum were gathered by tape stripping (Protect Label typeA; AsOne, Osaka, Japan) to measure the cathepsin L-like protease activity level (an important enzyme for keratinocyte differentiation). The tape-attached corneocytes were immersed in 0.2 M Tris-HCl buffer (pH 6.8) containing 0.5 mM Z-Val-Val-Arg-MCA synthetic peptide (substrate for cathepsin S/L; Peptide Institute Inc., Osaka Japan), 2% (v/v) N,N-dimethylformamide, and 0.05% (w/v) Triton-X100 (both from Wako Pure Chemical Industries, Osaka, Japan), for 2 h at 37°C. Then the fluorescence intensity (excitation wavelength 380 nm, emission wavelength 445 nm) of the buffer was measured using Spectra Max Gemini (Molecular Devices, Sunnyvale, CA, USA). Meanwhile, the tapes were immersed in BCATM Protein Assay Reagent (Thermo Scientific, Chicago, IL, USA) for 30 min at room temperature. Then the reagent's absorbance at 492 nm was determined using a Bio Kinetics Reader (Bio-Tek; Winooski, VT, USA), and protein levels were calculated. The individual levels of cathepsin L-like protease activity in stratum corneum were expressed as fluorescence intensity per protein (μ.g).
Measurement of phenols by high-performance liquid chromatography (HPLC)
Phenols in serum were measured by a modified HPLC method as described by Niwa (Citation7). Standard solutions were prepared at concentrations in the range 0.1–50.0 nmol/ml phenol (Nacalai Tesque, Kyoto, Japan) and p-cresol (Wako Pure Chemical Industries). As the internal standard, p-isopropylphenol (Tokyo Chemical Industry, Tokyo, Japan) was used at 0.5 μmol/ml.
The chromatography was done with Waters 2690 Separation Module Alliance with the routine column, i.e. C18-Octa Decyl Silica column 4.6 mm × 150 mm (F-411; Shodex, Tokyo, Japan), and the Waters 2475 Multi λFluorescence Detector (Nihon Waters, Tokyo, Japan). The HPLC conditions were the same as described by Niwa (Citation7).
Cell culture
Epidermal keratinocytes from normal adult female breast skin (Epidercell NHEK(F); Kurabo Biomedical Business, Osaka, Japan) were grown at 37°C (5% CO2, humidified atmosphere) in keratinocyte growth medium supplemented with 10 μg/ml insulin, 0.1 ng/ml human epidermal growth factor, 0.5 μg/ml hydrocortisone, 0.4% (v/v) bovine pituitary extract, 50 μg/ml gentamicin, and 50 ng/ml amphotericin B (Humedia-KG2; Kurabo Biomedical Business).
Subconfluent cells were subjected to 20 nmol/ml phenol (Nacalai Tesque), 20 nmol/ml p-cresol (Wako Pure Chemical Industries), or sterilized water in equal volumes then cultured for 3 days. On day 3, the cells were incubated with fresh culture medium containing 10% (v/v) water-soluble tetrazolium salt (WST-8; Dojindo, Kumamoto, Japan) for 2 h at 37°C. Then the medium's absorbance at 450 nm was determined using a Bio Kinetics Reader (Bio-Tek), and percent control for the phenols was calculated.
Western blot analysis
All materials for which the origin of acquisition is not indicated below were obtained from Sigma (St Louis, MO, USA).
To obtain keratinocyte extracts, 106 monolayer-cultured keratinocytes were harvested in a phosphate-buffered saline (pH 7.4) without CaCl2 and MgCl2 containing an excess of protease inhibitor cocktail (Complete Mini; Roche Diagnostics KK, Tokyo, Japan). The cells were suspended with fivefold RIPA Buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) containing an excess of protease inhibitor cocktail (Complete Mini), and incubated on ice for 30 min. A supernatant cell lysate was collected by centrifuging (10 000 g, 10 min) to pellet the cell debris. Protein levels in the cell lysate were determined using BCATM Protein Assay Reagent (Thermo Scientific).
The cell lysate samples were dissolved in an equal volume of sodium dodecyl sulfate (SDS)-sample buffer containing 2.3% (w/v) SDS, 10% (w/v) glycerol, 5% (v/v) 2-mercaptoethanol, and 10 μg/ml bromophenol blue in 125 mM Tris-HCl (pH 6.8), and heated at 100°C for 5 min. These samples were subjected to SDS-PAGE (10% w/v acrylamide) in an equal quantity of protein, and transferred onto a polyvinylidene fluoride membrane (ImmobilonTM; Millipore, Billerica, MA, USA). The membrane was soaked in a 20 mM Tris-HCl (pH 7.4) buffer containing 0.15 M NaCl (TBS) supplemented by 20 mg/ml bovine serum albumin (BSA) overnight at 4°C, and incubated with a 500-fold diluted monoclonal antibody to Cytokeratin10 (Progen Biotechnik, Heidelberg, Germany) for 2 h at room temperature. Then, the antibody treated-membrane was washed with TBS containing 0.05% (w/v) Tween 20 to remove excess antibodies, and incubated with anti-mouse IgG conjugated with alkaline phosphatase (Promega, Madison, WI, USA). Antigens were made visible by the enzymatic reaction of alkaline phosphatase with 5-bromo-4-chloro-3-indolylphosphate and nitro-blue tetrazolium (Promega), and analyzed using an image analyser (ScnImage).
Statistical analysis
Spearman's rank correlation method was used to determine the significance of correlation between phenol levels and corneocyte size. The Wilcoxon's signed rank sum test was used to determine the significance of variation before and after administration of prebiotic beverage. Experimentation using monolayer-cultured cells was carried out as three independent trials, percent control was calculated, and its mean and SD were plotted. The Tukey test determined statistical significance of variations among three groups (Control, Phenol, and Cresol in and ). Statistical significance was set at p < 0.05.
Ethics and consent
All experiments were performed in accordance with the latest amendment of the Helsinki Declaration. The protocol and informed consent form were approved by the Yakult Central Institute's Ethical Committee for Human Experimentation. All participants provided written informed consent.
Results
Correlation between levels of serum phenols and corneocyte size in healthy women
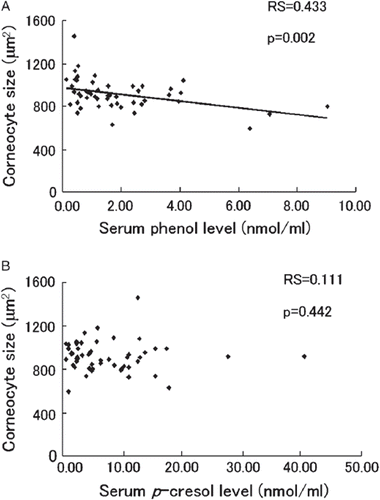
Among the 50 healthy female volunteers, a significant inverse correlation was seen between phenol levels and corneocyte size (, Spearman's rank-correlation coefficient: RS = 0.433, two-tailed p value: p = 0.002). The correlation between p-cresol levels and corneocyte size was not significant (, RS = 0.111, p= 0.442).
Figure 1. Correlation between levels of serum phenols and corneocyte size in healthy women. Serum (A) phenol and (B) p-cresol levels and corneocyte size (taken as the mean size of 30 measured cells/human) of inner forearm skin were measured in 50 healthy female volunteers (aged 21–60 years). Spearman's rank correlation between phenol levels and corneocyte size was analyzed. Each individual's value is plotted as a diamond. Spearman's rank-correlation coefficient (RS) and the two-tailed p value (p) are indicated.
Variation of serum phenol levels and stratum corneum condition in healthy women given a prebiotic beverage
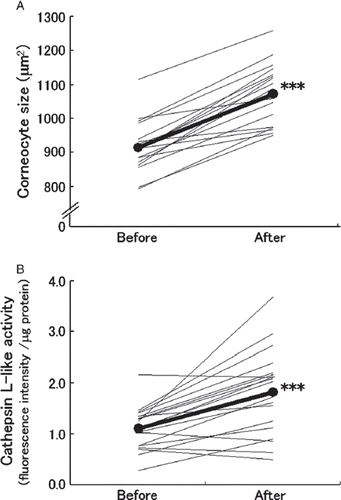
The 19 healthy female volunteers given the prebiotic beverage showed decreased mean levels of both serum phenol () and serum p-cresol (). However, only changes in p-cresol levels were significant (p < 0.001). Both corneocyte size () and cathepsin L-like protease activity level () increased significantly from before the prebiotic beverage (p < 0.001) in these women.
Figure 2. Variation in levels of serum phenols in healthy women administered a prebiotic beverage. Nineteen healthy female volunteers (aged 21–39 years) were administered a prebiotic beverage containing 3.0 g galacto-oligosaccharides and 5.6 g polydextrose for 3 weeks. The serum (A) phenol and (B) p-cresol levels at week 0 (before) and week 3 (after) were measured and each individual variation is plotted as a fine line. Mean values are plotted as black circles connected by a thick line. The statistical significance of variation between before and after determined by the Wilcoxon's signed rank sum test is indicated as ***p < 0.001.
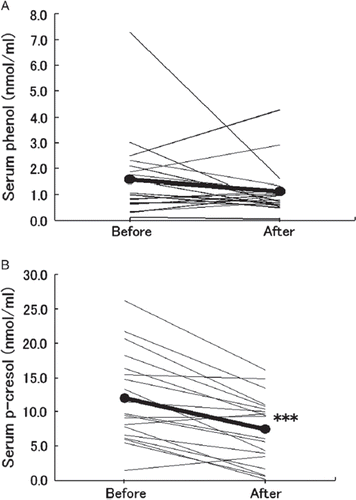
Figure 3. Variation in skin condition of healthy women administered a prebiotic beverage. Nineteen healthy female volunteers (aged 21–39 years) were administered a prebiotic beverage containing 3.0 g galacto-oligosaccharides and 5.6 g polydextrose for 3 weeks. (A) The corneocyte size (taken as the mean size of 30 measured cells/human) of inner forearm skin was measured at week 0 (before) and week 3 (after). (B) At identical intervals, cathepsin L-like protease activity in stratum corneum of inner forearm skin was measured. Each individual variation is plotted as a fine line. Mean values are plotted as black circles connected by a thick line. The statistical significance of variation between before and after analyzed by the Wilcoxon's signed rank sum test is indicated as ***p < 0.001.
In vitro experiment
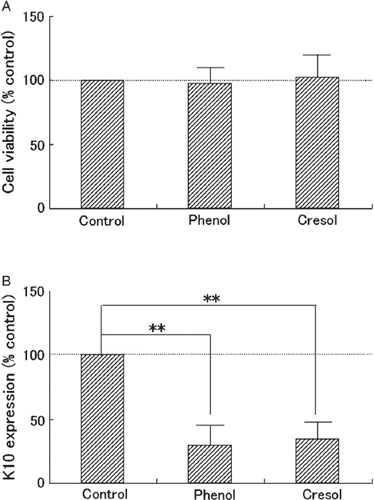
Neither phenol nor p-cresol significantly affected cell viability (), whereas both phenol and p-cresol significantly (p < 0.01) reduced K10 expression in cultured keratinocytes ().
Figure 4. Physiological effect of phenols on cultured keratinocytes. Monolayer-cultured normal human epidermal keratinocytes were subjected to 20 nmol/ml phenol (Phenol), 20 nmol/ml p-cresol (Cresol), or distilled water (Control) in equal volumes for 3 days. On day 3, (A) cell viability indicated by tetrazolium salt color reaction to metabolism, and (B) keratin 10 protein (K10) expression level indicated by western blot analysis were measured. Three independent experiments were carried out. Percent control for each was calculated and is plotted as mean and SD for the three. The statistical significance of differences observed among the three groups was determined by the Tukey test and is indicated as **p < 0.01.
Discussion
Our previous experiment (Citation6) using female hairless mice suggested that phenols produced by gut bacteria are absorbed, distributed by the blood, and then accumulate in the skin. We assumed that phenols accumulated in the skin might cause incomplete cornification, reflected by decreased corneocyte size. Initially, to determine if the same phenomenon applied to humans, Spearman's rank correlation between serum phenol levels and corneocyte size was determined for 50 female volunteers.
Comparable groups in their 20s to 50s were selected, as we thought age was an important factor affecting both intestinal environment and skin condition. It was further assumed that many lifestyle factors affected both intestinal environment and skin condition. However, because we could not find any lifestyle factors that significantly correlated with serum phenol levels or corneocyte size (data not shown), we excluded these parameters from further analysis. A larger population would bring more conclusive results.
Results showed a significant inverse correlation between phenol level and corneocyte size. Considering that the existence of phenols in a mammal's body is due to gut bacterial activity (Citation8), these results suggest that phenol produced by gut bacteria and distributed by blood causes incomplete cornification of the skin.
Two unexpected results emerge from this trial: 1) we found no significant correlation between p-cresol level and corneocyte size; and 2) we found no significant correlation between phenol levels and the b* value (an indicator of a yellowish dullness) of the inner forearm skin (data not shown), which was detected in our previous mouse experiment (Citation6). We assume that these two matters are due to individual variability of the human volunteers' skin. Because human skin color and corneocyte size vary more than that of inbred strain mouse skin, slightly fluctuating phenol levels in a range of healthy volunteers might not affect the skin in a detectable manner. Furthermore, our previous study (Citation6) indicated that after intraperitoneal administration of phenol in mice, corneocytes were significantly smaller than in mice given p-cresol, indicating that phenol has a stronger influence than p-cresol on corneocyte size. Thus, it was expected that a significant inverse correlation between phenol level and corneocyte size would be observed.
Because undesirable factors that affected skin condition (dryness, dullness, elasticity) appeared to increase with the age of the women volunteers (data not shown), only the younger women (in their 20s–30s) were selected for the prebiotic beverage administration trial. After a 3-week probiotic-, prebiotic-, and antibiotic-free period, volunteers were orally administered a prebiotic beverage containing galacto-oligosaccharides daily for 3 weeks; this beverage had been suspected of potentially affecting the intestinal environment (4,9). On day 22, the mean value of all subjects' serum phenol and p-cresol levels decreased. The decrease in p-cresol level was significant. At least three factors could explain this result: 1) the phenols-producing bacterial population was reduced by an increase in prebiotics assimilating non-phenols-producing bacteria such as bifidobacteria; 2) phenols-producing activity in intestinal bacteria was reduced by the pre-biotics' effects; and 3) phenols concentration in the gut was reduced because of the additional bowel movements induced by dietary fiber (9–11). Although understanding the mechanism of the effect of the prebiotic beverage is important, in this study our aim was limited to determining whether changes in serum phenol levels were related to skin condition.
In the 19 subjects who ingested the prebiotic beverage in whom serum phenol levels were reduced, the mean value of corneocyte size and cathepsin L-like protease activity in the stratum corneum significantly increased. In addition to knowing that corneocyte size represents keratinocyte differentiation conditions (Citation12), cathepsin L activity can also be viewed as a good indicator of keratinocyte differentiation conditions, as it is known that proteolysis by cathepsin L activates transglutaminase 3, a key enzyme for producing stratum corneum (Citation13). From these results, we assume that decreased skin phenol levels improve keratinocyte differentiation conditions. That is, phenols accumulated in the skin may disturb normal keratinocyte differentiation.
To determine whether phenols directly affect keratinocyte differentiation, we performed an in vitro experiment using monolayer-cultured normal female human keratinocytes. Keratinocytes were subjected to 20 nmol/ml phenol or 20 nmol/ml p-cresol in culture medium.
In our previous mouse experiment (Citation6), we confirmed comparable levels of phenols in serum and skin (average levels for mice fed a tyrosine-enriched diet in which skin exhibited a significant dullness were 10.54 nmol/ml for serum phenol, 10.92 nmol/g for skin phenol, 20.30 nmol/ml for serum p-cresol, and 21.97 nmol/g for skin p-cresol). Although we cannot examine skin phenols levels in human experiments for ethical reasons, we suspect that levels of serum and skin phenols are comparable in humans. Furthermore, our previous experiments indicated that the upper limit of serum phenol levels in normal women was 20 nmol/ml (data not shown). A WST-8 assay indicated that 20 nmol/ml phenols is non-toxic for cultured keratinocytes (). Thus, we consider that 20 nmol/ml phenols is physiologically accceptable.
After exposure to phenols for 3 days, the cell viability and K10 expression level were measured. Keratins are major structural proteins synthesized in keratinocytes during proliferation and differentiation, and assemble into a web-like pattern to strengthen the barrier function of the stratum corneum. Keratins have some isotypes, including basal keratins K5 and K14, proliferation-associated keratins K6 and K16, inflammation-associated K17, and differentiation-associated keratins K1 and K10 (Citation14). A previous study (Citation15) showed that K10-deficient mice exhibit an extremely delicate epidermis (with delayed barrier repair function and impaired hydration). Another study (Citation16) showed that epidermolytic hyper-keratosis in humans was associated with spinous layer K10 defects, suggesting that K10 expression is important for normal epidermis composition. Based on these studies, K10 is often used as a marker of normal keratinocyte differentiation (Citation17).
In monolayer-cultured keratinocytes, more than 0.1 mM Ca2+ concentration of cultured medium induces cell differentiation, which is indicated by K10 expression (Citation18). In our experiment, cultivation of subconfluent normal human keratinocytes in culture medium containing 0.15 mM Ca2+ for 3 days enabled detection of K10 expression through western blot analysis. At the same time, both phenol and p-cresol in cultured medium significantly reduced K10 expression, indicating that phenols degrade the keratinocyte differentiation processes. Further investigation may reveal how phenols degrade K10 expression, and how they affect other differentiation processes.
All our results suggest that phenols produced by gut bacteria adversely affect keratinocyte differentiation in female human skin. It is said that normal keratinocyte differentiation is important in maintaining homeostasis of the epidermal architecture. In future experiments, we will look for a correlation between keratinocyte differentiation disturbances caused by phenol exposure and skin barrier dysfunction exhibited by trans-epidermal water loss. These future investigations will reveal any correlations between an undesirable intestinal environment and skin problems, and may aid in the development of novel treatments to enhance skin health through an improved intestinal environment.
Acknowledgment
The authors thank all volunteers for their participation in these trials. We also acknowledge Dr Yuri Sugiura for her medical advice and support in the trials.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ara K, Meguro S, Hase T, Tokimitsu I, Otsuji K, Kawai S, . Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in human and rats. Microb Ecol Health Dis 2002;14:4–13.
- Bakke OM. Studies on the degradation of tyrosine by rat caecal contents. Scand J Gastroenterol 1969;4:603–8.
- Smith EA, Macfarlane G T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 1996;81:288–302.
- Preter VD, Geboes K, Verbrugghe K, Vuyst LD, Vanhoutte T, Huys G, . The in vitro use of the stable isotope-labelled biomarkers lactose-(15N)ureide and (2H4)tyrosine to assess the effect of pro- and prebiotics on the intestinal flora of healthy human volunteers. Br J Nutr 2004;92:439–46.
- Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 2006;21:351–66.
- Iizuka R, Kawakami K, Izawa N, Chiba K. Phenols produced by gut bacteria affect the skin in hairless mice. Microb Ecol Health Dis 2009;21:50–6.
- Niwa T. Phenol and p-cresol accumulated in uremic serum measured by HPLC with fluorescence detection. Clin Chem 1993;39:108–11.
- Bakke OM, Midtvedt T. Influence of germ-free status on the excretion of simple phenols of possible significance in tumour promotion. Experientia 1970;26:519.
- Ito M, Deguchi Y, Miyamori A, Matsumoto K, Kikuchi H, Matsumoto K, . Effect of administration of galactooli-gosaccharide on the human fecal microflora, stool weight and abdominal sensation. Microb Ecol Health Dis 1990;3:285–92.
- Kawakami K, Makino I, Asahara T, Kato I, Onoue M. Dietary galacto-oligosaccharides mixture can suppress serum phenol and p-cresol levels in rats fed tyrosine diet. J Nutr Sci Vitaminol 2005;51:182–6.
- Vuksan V, Jenkins AL, Jenkins DJ, Rogovik AL, Sievenpiper JL, Jovanovsuki E. Using cereal to increase dietary fiber intake to the recommended level and the effect of fiber on bowel function in healthy persons consuming North American diets. Am J Clin Nutr 2008;88:1256–62.
- Lee S, Park YK, Kim YK, Kang JS. An experimental study on corneocytes of acutely and chronically irritated skin. Arch Dermatol Res 1983;275:49–52.
- Cheng T, Hitomi K, Vlijmen-Willems IMJJV, Jongh GJ, Yamamoto K, Nishi K, . Cystain M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystain M/E in epidermal cornification. J Biol Chem 2006;281:15893–9.
- Ehrhardt P, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol 2008;17:1063–72.
- Jensen JM, Schutze S, Neumann C, Proksch E. Impaired cutaneous permeability barrier function, skin hydration, and sphingomyelinase activity in keratin 10 deficient mice. J Invest Dermatol 2000;115:708–13.
- Roop D. Defects in the barrier. Science 1995;267:474–5.
- Ekanayake-Mudiyanselarge S, Aschauer H, Schmook FP, Jensen JM, Meingassner JG, Proksch E. Expression of epidermal keratins and the cornified envelope protein involucrin is influenced by permeability barrier disruption. J Invest Dermatol 1998;111:517–23.
- Hennings H, Kruszewski FH, Yuspa SH, Tucker RW. Intra-cellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcino-genesis 1989;10:777–80.
