Abstract
Lupus nephritis is one of the most serious complications of systemic lupus erythematosus and manifests with considerable phenotypic and histological heterogeneity. In particular, diffuse proliferative lupus nephritis (DPLN) and membranous lupus nephritis (MLN) represent morphologic forms that are polar opposites. DPLN is associated with autoimmune responses dominated by Th1 immune response associated with high levels of interferon (IFN)-γ. In contrast, a Th2 cytokine response is associated with the pathogenesis of MLN. MRL/lpr mice develop human LN-like immune complex-associated nephritis and provide a suitable histological model for human DPLN. Infection with Schistosoma mansoni skewed a Th2-type immune response induction and IL-10 in MRL/lpr mice, drastically changing the pathophysiology of glomerulonephritis from DPLN to MLN accompanied by increased IgG1 and IgE in the sera. T cells in 32-week-old MRL/lpr mice infected with S. mansoni expressed significantly more IL-4 and IL-10 than T cells of uninfected mice; T cells with IFN-γ were comparable between infected and uninfected MR/lpr mice. Thus, the helminthic infection modified the cytokine microenvironment and altered the pathological phenotype of autoimmune nephritis.
Introduction
Systemic lupus erythematosus (SLE) is a complex multisystem disease, characterized by massive overproduction of polyclonal antibodies and impaired clearance of immune complexes. Tissue damage results from deposition of pathogenic immune complexes, which trigger interacting cascades of inflammation, vasculopathy and sclerosis. Lupus nephritis [Citation1] manifests with considerable phenotypic and histological heterogeneity. The classification scheme for LN was proposed by a joint committee of the International Society of Nephrology and the Renal Pathology Society [Citation2,Citation3]; where classes I through VI were established. Nephrotic syndrome is commonly associated with class IV or class V, which represent polar opposite morphologic forms. Class IV is called diffuse proliferative lupus nephritis (DPLN) and is characterized by diffuse global or segmental proliferative glomerulonephritis; class V is called membranous lupus nephritis (MLN) and is characterized by histopathologically diffuse, membranous nephropathy (MN). DPLN is the most common, severe and important form of LN. Patients with DPLN typically present with the most active and severe clinical features. These patients often have high anti-DNA antibody titers, low serum complement levels and very active urinary sediment with erythrocytes and cellular casts on urinalysis [Citation4,Citation5]. They usually have the least favorable prognosis in older series, eventually progressing to renal failure despite aggressive treatment [Citation4,Citation5]. However, less than 60% of MLN patients show such low serum complement and elevated anti-DNA antibody titer at presentation [Citation6], and MLN patients have generally more favorable prognosis than DPLN. The formation of circulating immune complexes and their deposition in glomeruli followed by complement activation is important for glomerular damage in LN. It is unclear why the same mechanism in the same disease yields different clinical and histological phenotypes, such as DPLN and MLN. Although marked heterogeneity in SLE with renal manifestations can be explained by genetic, pathogenic and environmental diversities, it is unclear whether any of these components is a primary or major contributor to the pathology of LN [Citation7]. The pathogenesis of DPLN is associated with a predominance of Th1 cytokines, indicating that DPLN develops in a Th1-dominant immune response. The renal tissue of DPLN patients shows increased levels of Th1 cytokines, including interferon (IFN)-γ [Citation8,Citation9]; peripheral blood lymphocytes (PBLs) from DPLN patients also produce elevated levels of IFN-γ. Furthermore, the ratio of IFN-γ vs. IL-4 production correlates positively with the histological activity index of the nephritis [Citation9]. MLN is a representative of secondary MN. MN is characterized by glomerular deposition of IgG and complement in the glomerular capillary walls, observable by immunofluorescence examination. In primary MN, the target antigen is an M-type phospholipase A2 receptor (PLA2R) expressed in normal podocytes, and the antibodies against a conformation-dependent epitope in PLA2R were predominantly of the IgG4 subclass (human Th2 subclass) [Citation10]. No signs of delayed hypersensitivity can be observed in the tissue of MN. In other words, intraglomerular and interstitial accumulation of T cells, macrophages and fibrin are consistently negative in the tissue of MN. Based on this indirect evidence, MN is generally considered an immune-complex-mediated disease with predominant Th2 nephritogenic immune response [Citation11,Citation12]. PBLs from MN patients show a decreased ratio of IFN-γ vs. IL-4 production [Citation13], and the similar characteristics observed in PBLs from MLN patients suggested that a Th2 cytokine response is associated with the pathogenesis of MLN [Citation8].
MRL/MpJ-lpr/lpr (MRL/lpr) mice have a mutation in the gene encoding Fas (lpr) and are particularly valuable for the investigation of SLE pathogenesis [Citation14]. The MRL background is responsible for the development of autoimmune kidney disease, and the Faslpr mutation converts a latent, mild nephritis observed in MRL/MpJ mice into a rapid, fulminant condition, with a 50% mortality rate at six months of age [Citation15]. The central features of this glomerulonephritis are accumulation of monocytes/macrophages within glomeruli, infiltration of T cells in the periglomerular interstitium, proliferation of glomerular mesangial cells and expansion of the mesangial extracellular matrix. MRL/lpr mice exhibit severe pan-isotypic hypergammaglobulinemia, autoantibody production and lymphadenopathy, which are also important to consider as a model for human DPLN. Moreover, like human DPLN, the contribution of Th1 responses to the development of DPLN has been well characterized in MRL/lpr nephritis [Citation16–18]. In addition, WSX-1-deficient MRL/lpr mice provide a spontaneous animal model for human MLN. WSX-1 is IL-27 receptor α, and IL-27 signaling plays a critical role in the initial mounting of proper Th1 responses during Leishmania major infection in C57BL/6 wild-type mice [Citation19] and during the development of autoimmune DPLN in MRL/lpr mice. Thus, WSX-1 deficiency in MRL/lpr mice skewed the ongoing immune responses to autoantigens within the mice from Th1-dominant to Th2-dominant, causing drastic changes in the pathological features of glomerulonephritis from DPLN to MLN, which finally changed the outcome of the autoimmune disease [Citation20]. Thus, WSX-1-deficient MRL/lpr mice are a suitable spontaneous animal model for human MLN.
S. mansoni is a helminthic parasite and a causative agent for intestinal schistosomiasis. It is estimated that 600–779 million people are at risk of infection [Citation21,Citation22]. The cercariae, larvae of S. mansoni, infect the definitive host by penetrating the skin and transform to schistosomula, which migrate to and dwell in mesenteric and hepatic portal blood vessels, where they develop into sexually mature adults and lay eggs around the mesenteric blood vessels. The eggs of S. mansoni are deposited on the endothelial lining of the venous capillary walls. Some of the eggs pass through the wall of the intestine to the lumen and are released into the environment with the feces; the rest of the eggs are swept into hepatoportal circulation and trapped in hepatic sinusoids, causing inflammation, vascular obstruction and fibrosis [Citation23]. S. mansoni is distinct from Schistosoma haematobium, which causes urinary schistosomiasis.
It is well known that eggs of S. mansoni induce a strong Th2 immune response together with IL-10 [Citation24]. In this study, we infected MRL/lpr mice with S. mansoni and examined the histopathological phenotype of glomerulonephritis to understand the LN phenotype in the context of the systemically induced Th2 immune response during S. mansoni infection.
Materials and methods
Schistosoma mansoni infection
A Puerto Rican strain of S. mansoni (NIH-Sm-PR-1 strain) was maintained by passage through gerbils and Biomphalaria glabrata snails. Female MRL/lpr mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and subcutaneously infected with 50 cercariae S. mansoni at the age of approximately 8 weeks. Fecal samples were collected regularly over 40 weeks, and eggs were confirmed. It was demonstrated that host immune responses change dramatically during S. mansoni infection; in the early phase, Th1-related responses are induced, whereas during the late phase Th2 reactions dominate [Citation25].
Mice were housed in a specific pathogen-free (SPF) facility in the Laboratory of Animal Experiments of Nagasaki University. All experiments were approved by the Institutional Animal Research Committee of Nagasaki University and conformed to the animal care guidelines of the American Physiologic Society. Spleens and kidneys were harvested at the time of sacrifice and processed for immunology and microscopy.
Serologic analysis and urinalysis
For serum Ig determinations, ELISA was performed using the following Abs: immunoenzymetric assay kits for the measurement of total mouse IgG, IgG1 and IgG2a (Cygnus Technologies Inc., Southport, NC); the level of anti-dsDNA Ab was measured using a commercially available ELISA kit (Shibayagi Co. Ltd., Gunma, Japan). Determination of serum cytokine levels was performed using Cytometric Bead Array Kit (BD Biosciences Co. Ltd., Franklin Lakes, NJ), according to the manufacturer's instructions. For urinalysis, 100 µl of urine was taken from each mouse and was analyzed for protein and creatinine levels (SRL Inc., Tokyo, Japan). For brief quasi-quantification of 24-h protein in the urine, the urinary protein:creatinine ratio was calculated for each sample.
Histopathological and immunohistopathological studies
Mouse kidneys were fixed in 10% formalin for 24 h at 4 °C. Paraffin sections (4 µm) were stained with hematoxylin and eosin, periodic acid Schiff stain or periodic acid-methenamine silver (PAM). For immunohistochemical staining, kidneys were snap-frozen in optimal cutting temperature compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan). To detect IC deposits, cryostat sections (2 µm) were fixed in chilled acetone and stained with fluorescein isothiocyanate (FITC)-conjugated goat polyclonal anti-mouse IgG Abs (SYSMEX BioMerieux Co., Ltd., Tokyo, Japan), a FITC-conjugated goat anti-mouse IgG1 Ab and FITC-conjugated goat anti-mouse IgG2a Ab (Southern Biotechnology Associates, Inc., Birmingham, AL). For negative controls, sections were treated with normal goat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For electron microscopy, kidney tissues were fixed in 3% glutaraldehyde, post-fixed in osmium tetroxide, dehydrated in a graded alcohol series and embedded in Epon812 before serial cutting. Ultrathin sections (60–80 nm) were double-stained with uranyl acetate and Reynold's lead citrate.
In vitro induction of cytokine production by CD4+T cells
CD4+T cells were purified from splenic single cell suspensions using FITC-conjugated monoclonal antibody to CD4 molecule (GK 1.5) and anti-FITC microbeads (Miltenyi Biotec K.K., Tokyo, Japan). Purified CD4+T cells (2.5 × 105/200 µl) were stimulated with 5 µg/mL anti-CD3 + 2.5 µg/mL anti-CD28 in the presence of GolgiPlug and GolgiStop (BD PharMingen Co. Ltd.) for 5 h. Cells were fixed and permeabilized with the Cytofix/Cytoperm Plus kit (BD Biosciences Co. Ltd.) according to the manufacturer's directions. Cells were stained with FITC or PE-conjugated monoclonal Ab against each cytokine. Purified CD4+T cells (2.5 × 105/200 µl) were also stimulated with 5 µg/mL anti-CD3+2.5 µg/mL anti-CD28 for 48 h, and culture supernatants were analyzed for cytokine production using the Mouse Cytokine ELISA Plate Array (Signosis, Inc., Santa Clara, CA) according to the manufacturer's directions. Samples in 96-well microtiter plates were analyzed on a LAS3000 (FujiFilm Ltd., Tokyo, Japan) and the chemiluminescent intensity of each sample was evaluated by Image Gauge ver. 4.0 (FUJIFILM Software, Yokohama, Japan). The ratios of each sample to a blank of intensity per mm3 were obtained. The production of TGF-β was analyzed with a mouse TGF-β1 DuoSet ELISA Development system (R&D Systems, Inc., Minneapolis, MN).
Real-time quantitative PCR and TaqMan primers and probes
Total RNA was prepared from the kidneys with an RNeasy Mini Kit (QIAGEN, Valencia, CA) and quantitative cDNA amplification was performed according to the manufacturer's instructions. All samples were stored at –80 °C before use. The examined cytokines included IFN-γ, IL-12p35, IL-4, IL-5, IL-10, TGF-β, IL-17A and IL-6. Foxp3 is a transcription factor specific for CD4+CD25+Foxp3+Tregs and Foxp3 mRNA was also evaluated. To provide a meaningful comparison between samples, we calculated the amount of PCR products relative to the amount of β-actin in each sample. Cytokine levels were measured using TaqMan-PCR and an ABI prism 7700 sequence detection system (PerkinElmer Japan Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions (Applied Biosystems Japan Ltd., Tokyo, Japan). Oligonucleotide primers and probes were designed using Primer Express (Applied Biosystems Japan Ltd.). Primer and probe sequences included IFN-γ; Mm99999071_m1, IL-12; Mm00434165_m1, IL-4; Mm00445260_m1, IL-5; Mm00439646_m1, IL-6; Mm99999064_m1, IL-17; Mm00439619_m1, IL-10; Mm00439616_m1, TGF-β; Mm00441726_m1, Foxp3; and Mm00475157_g1.
Statistical analyses
To calculate survival rates, Kaplan–Meier analysis was performed. Quantitative data are expressed as the mean ± SD. The Mann–Whitney rank sum test was performed to analyze differences between groups. A level of p < 0.05 was considered statistically significant.
Results
Alteration of kidney pathology in S. mansoni-infected MRL/lpr mice
MRL/lpr mice were kept in a SPF environment after birth; S. mansoni infection was initiated when the mice were 8 weeks old (wo). Successful infection was confirmed by detection of eggs in the feces. We monitored survival rate of infected MRL/lpr mice (). These mice expired at a similar rate; 50% dead by 24 weeks after birth in both groups. Blood urea nitrogen in S. mansoni-infected MRL/lpr mice was significantly lower than in uninfected MRL/lpr mice at 32 wo (). DPLN deteriorated with age in uninfected MRL/lpr mice at 16, 24 and 32 wo (), with glomerular endocapillary proliferation, including mesangial cells and endothelial cells, with infiltrating mononuclear and polymorphonuclear leukocytes. In contrast, MRL/lpr mice infected with S. mansoni showed mild inflammatory cell infiltration and slight proliferative lesions in the glomeruli even at 32 wo. The degree of proliferative changes was significantly lower in infected mice, but pathological changes still occurred in the kidney. In fact, most of the glomeruli observed in infected 32 wo MRL/lpr mice showed diffuse thickening of the basement membrane. In infected MRL/lpr mice, glomerular staining with PAM clearly revealed generalized diffuse thickening of peripheral capillary walls, with spike-like alterations of basement membranes (). At 32 wo, all infected MRL/lpr mice showed membranous glomerular changes, while such alterations were not observed in the uninfected MRL/lpr mice. Electron microscopy revealed deposition of electron-dense materials in the subepithelial basement membrane. In infected MRL/lpr mice, mesangial hypercellularity and deposition and small subendothelial depositions were seen (), all of which are characteristics of glomeruli with membranous changes. Because Ig deposition is one of the hallmarks of glomerulonephritis, we used immunofluorescence staining to detect each Ig subclass in the kidneys of uninfected and infected MRL/lpr mice. Although Ig deposition was observed in both groups, the patterns of deposition were distinct. In uninfected MRL/lpr mice, IgG1 (mouse Th2 subclass) and IgG2a (mouse Th1 subclass) were equally deposited until week 24; then IgG2a became the predominant isotype in mesangial lesions and along the glomerular capillary walls at 32 wo (). In contrast, IgG1 was predominantly deposited in the glomeruli of infected 24 wo MRL/lpr mice. In the glomerular capillary walls of infected mice, widespread, discrete and granular depositions were exhibited at 32 wo; this is a major characteristic of glomeruli with membranous changes (). These data suggest S. mansoni infection altered the phenotype of autoimmune nephritis in MRL/lpr mice from that with the features that of human DPLN to MLN.
Figure 1. Similar survival of Schistosoma mansonie infected female MRL/lpr mice compared with uninfected female MRL/lpr mice. The cumulative survival of MRL/lpr mice was monitored (n; 20 per group). There was no difference in the survival rate between two groups.
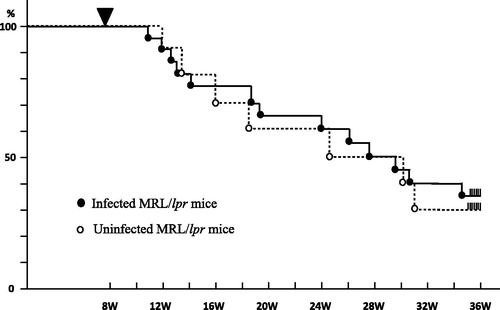
Figure 2. Altered histopathology of glomerulonephritis in MRL/lpr mice uninfected (1a) and infected (1b) with S. mansoni. (A) and (B). Light microscopic images of the kidney (PAM). (A) A representative glomerulus; original magnification, × 400. (B) High-zoom feature of basement membrane, × 800. (C) and (D), Electron micrograph of the glomerulus. (C) A feature of mesangial region, × 4000 (D), a feature around glomerular basement membrane (GBM), × 4000. E and F, Immunostaining of the glomerulus. (E) With anti-mouse IgG1 antibody, × 400 (F) with anti-mouse IgG2a antibody, × 400. 16 wo: (A) Mesangial cell proliferation was mild. (B) GBM thickening was not seen. Foot processes were normal. (C) Mesangial cells were slightly increased. (D) No abnormalities in either podocytes or GBM. Scant staining of IgG1 (E) and IgG2a (F) was recognized.24 wo: (A) Mesangial cell proliferation was moderate. (B) GBM thickening was not seen. Foot processes were normal. (C) Mesangial cells were slightly increased. (D) No abnormalities were seen in podocytes or GBM. IgG1 (E) and IgG2a (F) were equally lightly stained. 32 wo: (A) Mesangial cell proliferation associated with crescent formation was clearly recognized. (B) GBM thickening was recognized. (C) Subendothelial dense deposits were clearly recognized with mesangial cell proliferation. (D) Remarkable subendothelial deposition was observed. IgG1 (E) and IgG2a (F) were clearly stained. Notably, IgG2a (F) was stronger than IgG1 (E).(b) 16 wo: (A)–(F) Findings were similar in infected and uninfected mice. 24 wo: (A)–(D) Findings were similar in infected and uninfected mice. IgG1 (E) was clearly stained around GBM, but IgG2a (F) was not. 32 wo: (A) GBM thickening was clearly recognized. Mesangial cells were recognized, but milder than in uninfected mice. (B) GBM thickening with spike formation was apparent. (C) Subendothelial dense deposits were not recognized. (D) Subepithelial dense deposits with GBM thickening and foot process effacement were clearly recognized. IgG1 (E) was more apparent than at 24 wo. IgG2a (F) was also stained.
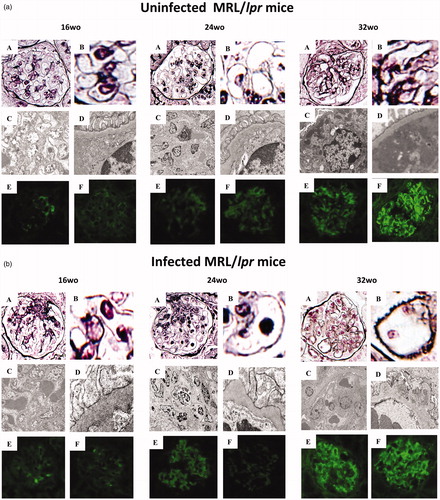
Table 1. Clinical manifestations and serum chemistry in S. mansoni uninfected and infected female MRL/lpr mice (N = 3/each group).
Elevation of Th2-associated Ig in the sera of infected MRL/lpr mice
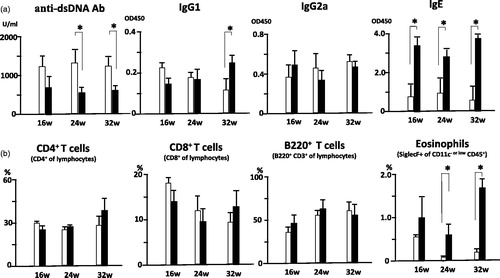
Autoantibodies play a crucial role during development of autoimmune nephritis and were measured over time. At 24 and 32 wo, the levels of anti-dsDNA Abs were significantly lower in infected MRL/lpr mice than in uninfected mice. The level and proportion of each serum Ig isotype reflects the cytokine microenvironment; these were also measured. Although there were no significant differences in total IgG and IgG2a (data not shown), the levels of IgG1 and IgE were significantly higher in infected MRL/lpr mice at 32 wo and throughout the experimental period, respectively (). These results strongly suggest that S. mansoni infection in the portal vein systemically biased even the autoimmune response in MRL/lpr mice toward Th2.
Figure 3. (a) Altered levels of serum Ig and autoantibodies in S. mansoni-infected MRL/lpr mice. Sera were collected from 16 wo, 24 wo and 32 wo infected and uninfected MRL/lpr mice, and levels of anti-dsDNA Ab and serum Ig were measured, as described in the Methods. Data are mean concentration ± SD of three mice per group. *p < 0.05 by unpaired t test, black bar; infected MRL/lpr mice and white bar; uninfected MRL/lpr mice. (b) Unaltered lymphocyte compartment in S. mansoni-infected MRL/lpr mice. Spleens were removed from 16 wo, 24 wo, and 32 wo infected and uninfected MRL/lpr mice, and single cell suspensions were stained for surface markers, followed by flow cytometry. Significant increases of eosinophils were observed in 24 wo and 32 wo infected MRL/lpr mice compared to uninfected MRL/lpr mice. Data are mean percentage ± SD of three mice per group. *p < 0.05 by unpaired t test, black bar; infected MRL/lpr mice and white bar; uninfected MRL/lpr mice.
Unaltered lymphocyte compartment in infected MRL/lpr mice
MRL/lpr mice show massive lymphoid hyperplasia due to an accumulation of abnormal B220+CD3+ lymphocytes that cannot undergo apoptosis because of the Fas point mutation [Citation26]. Both infected and uninfected mice showed splenomegaly. Neither the proportions of B220+CD3+ cells in these animals () nor the numbers of CD4+ and CD8+ T cells were affected by S. mansoni infection (data not shown). The proportion of eosinophils, however, significantly increased in infected MRL/lpr mice vs. uninfected MRL/lpr mice at 24 and 32 wo, reinforcing the model of an augmented Th2 immune response in infected MRL/lpr mice ().
Increased IL-4, IL-10 and TGF-β production by S. mansoni infection
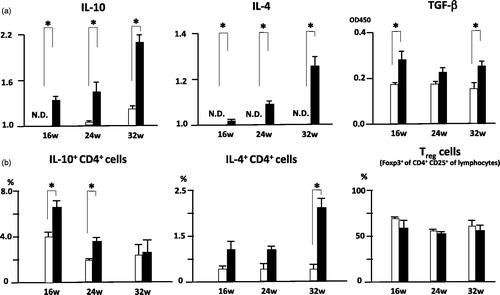
T cells from infected mice exhibit a primarily Thl-type immune response to larval antigens; oviposition triggers a strong Th2 response, which appears to be largely directed toward the eggs. As parasite eggs start to be produced from recently matured worms 6–8 weeks post-infection, the immune response takes on an entirely different character, becoming strongly polarized to Th2 [Citation27,Citation28]. To further characterize the altered immune responses in infected MRL/lpr mice, we examined cytokine production by CD4+ T cells isolated from spleens. As expected, CD4+ T cells derived from infected MRL/lpr mice produced significantly more IL-4 than similarly stimulated CD4+ T cells from uninfected MRL/lpr mice. Similar trends were observed for IL-10 and TGF-β (). They produced almost equal levels of IFN-γ (data not shown). The proportion of IL-4-producing cells was significantly higher in infected MRL/lpr mice than in uninfected mice at 32 wo. There were also significantly more IL-10-producing cells in infected MRL/lpr mice than in uninfected mice at 16 and 24 wo (). The proportions of IFN-γ or IL-17 producing CD4+ T cells were comparable between groups (data not shown).
Figure 4. (a) Altered IL-10, IL-4 and TGF-β production in in vitro stimulated CD4+ T cells of S. mansoni-infected MRL/lpr mice. Spleens were removed from 16 wo, 24 wo and 32 wo infected and uninfected MRL/lpr mice, and cells were stimulated with anti-CD3+anti-CD28 mAbs. Culture supernatants were analyzed for cytokine production using Mouse Cytokine ELISA Plate Array and mouse TGF-β ELISA kit as described in the Methods. Data are mean intensity and absorbance unit ± SD of three mice per group. *p < 0.05 by unpaired t test, black bar; infected MRL/lpr mice and white bar; uninfected MRL/lpr mice. (b) Altered IL-10 and IL-4 production in CD4+ T cells of S. mansoni-infected MRL/lpr mice. Spleens were removed from 16 wo, 24 wo and 32 wo infected and uninfected MRL/lpr mice, and intracellular cytokine staining was performed, followed by flow cytometry as described in the Methods. Data are mean percentage ± SD of three mice per group. *p < 0.05 by unpaired t test, black bar; infected MRL/lpr mice and white bar; uninfected MRL/lpr mice.
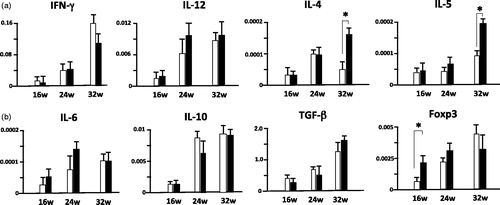
Increase of IL-4 and IL-5 mRNA in the kidneys of infected MRL/lpr mice at 32 wo
The amounts of IL-4 and IL-5 mRNA in the kidneys significantly differed between groups. The amount of IFN-γ mRNA in the kidneys was comparable in both groups throughout the experiment ().
Figure 5. Increased IL-4 and IL-5 mRNA expressions in the kidney of 36 wo S. mansoni-infected MRL/lpr mice vs. uninfected MRL/lpr mice. Data are the relative expression levels of various cytokines and Foxp3 mRNAs (normalized to β-actin mRNA expression) in the kidneys from infected and uninfected MRL/lpr mice. Data are mean level ± SD of 3 three mice per group. *, p < 0.05 by unpaired t test, black bar; infected MRL/lpr mice and white bar; uninfected MRL/lpr mice.
Discussion
Our most important finding was that S. mansoni infection completely changed the pathological features of glomerulonephritis in MRL/lpr mice. This phenotypic alteration from human DPLN-like to MLN-like disease was associated with a change in the cytokine microenvironment induced by S. mansoni infection.
The eggs of S. mansoni induce a strong Th2 response; this response to the eggs or soluble egg antigens (SEA) is characterized by high levels of IL-4, IL-5, IL-13 and IL-10 [Citation24,Citation29,Citation30]. IL-4 up-regulates fibroblast chemokine, matrix protein expression and collagen, suggesting IL-4 is a crucial cytokine for granuloma formation around eggs [Citation31] and reduces the cellular proliferative response to SEA [Citation32]. IL-5 induces eosinophilia and eosinophilic granulomas, as well as liver fibrosis [Citation33]. IL-10 is a major cytokine during the course of infection, negatively regulating Th1 and Th2 responses [Citation34].
DPLN is associated with the Th1 immune response, and we previously demonstrated that a lack of signaling through WSX-1 altered the phenotype of glomerulonephritis from DPLN to MLN. The immune response in WSX-1-deficient MRL/lpr mice was skewed to Th2, indicating the association between the Th2 microenvironment and pathological changes in the kidney of MRL/lpr mice observed in genetically manipulated mice. Instead of using knock-out mice in this study, we evaluated the effect of environmental changes induced by S. mansoni infection on the glomerulonephritis phenotype in MRL/lpr mice. The primary cause of death in MRL/lpr mice is renal insufficiency due to advanced nephritis (data not shown). In spite of the persistent infection, infected MRL/lpr mice survived at rates comparable to uninfected mice (), indicating that S. mansoni infection did not aggravate renal insufficiency and may have improved it. Light microscopy of kidney tissues in 32-wo infected MRL/lpr mice showed generalized diffuse thickening of peripheral capillary walls. PAM staining revealed a spike pattern (). Electron microscopy demonstrated the presence of electron-dense deposits in the subepithelial site with mesangial deposition. These findings are essentially identical to those observed in human MLN [Citation5]. Analysis of Ig subclasses in serum revealed elevated IgE throughout the experiment, and IgG1 increases with a concurrent decline in anti-dsDNA antibody at 32 wo in infected MRL/lpr mice (). Splenic cell surface markers did not significantly differ between infected and uninfected MRL/lpr mice with the exception of eosinophils at 24 and 32 wo (). Intracellular cytokine assays showed IL-10+CD4+ cells at 16 wo and 24 wo, and IL-4+CD4+ cells at 32 wo in infected MRL/lpr mice were significantly increased in comparison to uninfected MRL/lpr mice (). These results indicated Th responses of infected MRL/lpr mice skewed from Th1 to Th2 during persistent S. mansoni infection.
Thus, S. mansoni, which dwells in mesenteric and hepatic portal blood vessels and settles neither around nor in the kidney, completely change the phenotype of glomerulonephritis in MRL/lpr mice. These phenotypic alterations from human DPLN-like to MLN-like disease occurred during S. mansoni infection with a shift of Th balance from Th1 to Th2 in MRL/lpr mice, including immune responses against autoantigens from Th1- to Th2-dominant ones. We have demonstrated that disruption of the WSX-1 gene led altered the pathophysiology of glomerulonephritis in MRL/lpr mice [Citation20]. The initial mounting of Th1 responses depends on the function of the WSX-1 gene, which encodes the α subunit of IL-27R. In mice deficient for the WSX-1 gene, proper Th1 differentiation was impaired, and abnormal Th2 skewing was observed during infection with some intracellular pathogens [Citation19,Citation35,Citation36]. WSX-1–/– MRL/lpr mice developed human MLN-like disease with a predominance of IgG1 in glomerular deposits, accompanied by increased IgG1 and IgE in the sera. Similarly, disruption of EBV-induced gene 3, a subunit of IL-27, in MRL/lpr mice also altered the phenotype of glomerulonephritis from human DPLN-like to MLN-like disease [Citation37]. Thus, these reports suggested that loss of IL-27 favors Th2-type autoimmune responses, thus the association between Th1/Th2 balance and the development of human lupus nephritis. A recent study demonstrated that IL-27 is not only the most potent inducer of Th1 differentiation and IFN-γ production in naïve T cells but also possesses immunosuppressive properties and suppresses production of inflammatory cytokines [Citation38]. Therefore, although impaired IFN-γ production and aTh2 skewed immune response is observed in WSX-1 deficient mice, the real mechanism for the development of MLN in wild-type MRL/lpr mice is obscure. In this study, we examined the effect of S. mansoni infection on the development and phenotype of autoimmune nephritis in MRL/lpr. We found MRL/lpr did not show impairment of IFN-γ production (data not shown), but showed marked IL-10 and IL-4 production by S. mansoni infection ( and ). Human MLN is defined by regular subepithelial immune deposits producing a membranous pattern [Citation2,Citation39,Citation40], and is a representative of secondary MN. The coexistence of mesangial immune deposits and mesangial hypercellularity in most cases helps distinguish MLN from primary MN [Citation41]. It is notable that mesangial hypercellularity and deposition in the glomeruli was characteristic of infected MRL/lpr mice but was absent in WSX-1–/– MRL/lpr mice. Thus, from a histological point of view, MRL/lpr mice infected with S. mansoni more closely mimic human MLN than WSX-1–/– MRL/lpr mice, and this histological difference might be due to differences in IFN-γ production.
Helminthic infection is a characteristically potent inducer of Th2 cytokine responses including eosinophilia and IgE responses [Citation42], which may ameliorate atopic disorders in humans [Citation43]. Some experimental studies have shown that mice or rats infected with helminthes have reduced allergic responses [Citation44–47]. It was also demonstrated that IL-10 played a central role in resistance against allergic responses [Citation48,Citation49], and a number of IL-10-producing cells have been identified, including CD4+ T cells, CD4+CD25+ regulatory cells, macrophages and B cells [Citation45,Citation47]. Therefore, helminthes infection is associated with allergy suppression, and the proposed mechanism was called a “modified Th2 response” [Citation50,Citation51]. In this study, IL-10+ CD4+ T cells, not Treg cells, in the spleen of infected MRL/lpr mice were significantly increased in comparison to uninfected MRL/lpr mice at 16 and 24 wo; at 32wo, IL4+ CD4+ T cells were significantly increased (). These results indicated that S. mansoni infection might induce production of IL-4 and IL-10, but the Th1 response in MRL/lpr mice was not sufficiently suppressed. These microenvironmental changes might create the characteristic renal findings of infected MRL/lpr mice.
Lupus nephritis is a pathological condition triggered by tissue deposition of circulating immune complexes leading to release of inflammatory mediators, influx of inflammatory cells and clinically apparent disease. Phenotypic and histological heterogeneity are characteristic of LN, and complex immunological responses are included in the disease definition. Our study introduces the concept that Th1/Th2 balance of immune responses determines LN phenotype. We have demonstrated that a parasite infection that skews immune responses toward Th2 alters the histological features of glomerulonephritis in MRL/lpr mice from DPLN to MLN. Further study of Th balance in SLE patients should not only elucidate the pathogenesis of LN but also the evidence-based development of new therapies for LN.
Declaration of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
This study is supported, in part, by a grant for the Progressive Renal Research Projects from the Ministry of Health, Labor and Welfare of Japan (to T. S.) and grants from the Ministry of Education, Science, Technology, Sports and Culture of Japan to T. S. (#21591049) and H. N. (#21591048, #24591221).
Acknowledgements
We thank M. Miura and M. Hayashida for technical assistance and animal husbandry.
References
- Weening, J. J., V. D. D'Agati, M. M. Schwartz, et al. 2004. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 65: 521–530
- Weening, J. J., V. D. D'Agati, M. M. Schwartz, et al. 2004. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 15: 241–250
- Bomback, A. S., and G. B. Appel. 2010. Updates on the treatment of lupus nephritis. J. Am. Soc. Nephrol. 21: 2028–2035
- Appel, G. D. A., VD. 2007. Lupu nephritis – pathology and pathogenesis. In Dubois' Lupus Erythematosus. D. J. Wallace, and B. Hahn, eds. Lippincott Williams, Philadelphia, PA. p. 1049–1112
- Appel, G. B., D. J. Cohen, C. L. Pirani, et al. 1987. Long-term follow-up of patients with lupus nephritis. A study based on the classification of the World Health Organization. Am. J. Med. 83: 877–885
- Klinman, D. M., and A. D. Steinberg. 1995. Inquiry into murine and human lupus. Immunol. Rev. 144: 157–193
- Akahoshi, M., H. Nakashima, Y. Tanaka, et al. 1999. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 42: 1644–1648
- Masutani, K., M. Akahoshi, K. Tsuruya, et al. 2001. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 44: 2097–2106
- Beck L. H., Jr. R. G. Bonegio, G. Lambeau, et al. 2009. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361: 11–21
- Doi, T., M. Mayumi, K. Kanatsu, et al. 1984. Distribution of IgG subclasses in membranous nephropathy. Clin. Exp. Immunol. 58: 57–62
- Imai, H., K. Hamai, A. Komatsuda, et al. 1997. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 51: 270–276
- Masutani, K., M. Taniguchi, H. Nakashima, et al. 2004. Up-regulated interleukin-4 production by peripheral T-helper cells in idiopathic membranous nephropathy. Nephrol. Dial. Transplant. 19: 580–586
- Theofilopoulos, A. N., and F. J. Dixon. 1985. Murine models of systemic lupus erythematosus. Adv. Immunol. 37: 269–390
- Cohen, P. L., and R. A. Eisenberg. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9: 243–269
- Takahashi, S., L. Fossati, M. Iwamoto, et al. 1996. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J. Clin. Invest. 97:1597–1604
- Haas, C., B. Ryffel, and M. Le Hir. 1997. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J. Immunol. 158: 5484–5491
- Kikawada, E., D. M. Lenda, and V. R. Kelley. 2003. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology, J. Immunol. 170: 3915–3925
- Yoshida, H., S. Hamano, G. Senaldi, et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 15: 569–578
- Shimizu, S., N. Sugiyama, K. Masutani, et al. 2005. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1). J. Immunol. 175: 7185–7192
- Chitsulo, L., D. Engels, A. Montresor, and L. Savioli. 2000. The global status of schistosomiasis and its control. Acta Trop. 77:41–51
- Steinmann, P., J. Keiser, R. Bos, et al. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425
- Andrade, Z. A. 2004. Schistosomal hepatopathy. Mem. Inst. Oswaldo. Cruz. 99: 51–57
- Pearce, E. J. 2005. Priming of the immune response by schistosome eggs. Parasite Immunol. 27: 265–270
- Adachi, K., Y. Osada, R. Nakamura, et al. 2013. Unique T cells with unconventional cytokine profiles induced in the livers of mice during Schistosoma mansoni infection. PLoS One. 8: e82698
- Theofilopoulos, A. N., and D. H. Kono. 1999. The genes of systemic autoimmunity. Proc. Assoc. Am. Physicians. 111: 228–240
- Grzych, J. M., E. Pearce, A. Cheever, et al. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146: 1322–1327
- Pearce, E. J., P. Caspar, J. M. Grzych, et al. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173: 159–166
- Everts, B., G. Perona-Wright, H. H. Smits, et al. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206: 1673–1680
- Everts, B., L. Hussaarts, N. N. Driessen, et al. 2012. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 209: 1753–1767, S1
- Liu, X., T. Kohyama, H. Wang, et al. 2002. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L1049–L1056
- Correa-Oliveira, R., L. C. Malaquias, P. L. Falcao, et al. 1998. Cytokines as determinants of resistance and pathology in human Schistosoma mansoni infection. Braz. J. Med. Biol. Res. 31:171–177
- Reiman, R. M., R. W. Thompson, C. G. Feng, et al. 2006. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 74: 1471–9
- Mosmann, T. R. 1991. Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann. N. Y. Acad. Sci. 628: 337–344
- Sprecher, C. A., F. J. Grant, J. W. Baumgartner, et al. 1998. Cloning and characterization of a novel class I cytokine receptor. Biochem. Biophys. Res. Commun. 246: 82–90
- Chen, Q., N. Ghilardi, H. Wang, et al. 2000. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 407: 916–920
- Igawa, T., H. Nakashima, A. Sadanaga, et al. 2009. Deficiency in EBV-induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Mod. Rheumatol. 19: 33–41
- Yoshimura, T., Takeda, A., Hamano, S., et al. 2006. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4 + T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4 + T cells partially through STAT3-dependent mechanism. J. Immunol. 177: 5377–5385
- Donadio J. V., Jr. J. H. Burgess, and K. E. Holley. 1977. Membranous lupus nephropathy: a clinicopathologic study. Medicine (Baltimore). 56: 527–536
- Sloan, R. P., M. M. Schwartz, S. M. Korbet, and R. Z. Borok. 1996. Long-term outcome in systemic lupus erythematosus membranous glomerulonephritis. Lupus Nephritis Collaborative Study Group. J. Am. Soc. Nephrol. 7: 299–305
- Jennette, J. C., S. S. Iskandar, and F. G. Dalldorf. 1983. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 24: 377–385
- Fallon, P. G. 2000. Immunopathology of schistosomiasis: a cautionary tale of mice and men. Immunol. Today. 21: 29–35
- Yazdanbakhsh, M., P. G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis, Science. 296: 490–494
- Negrao-Correa, D., M. R. Silveira, C. M. Borges, et al. 2003. Changes in pulmonary function and parasite burden in rats infected with Strongyloides venezuelensis concomitant with induction of allergic airway inflammation. Infect. Immun. 71: 2607–2614
- Bashir, M. E., P. Andersen, I. J. Fuss, et al. 2002. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 169: 3284–3292
- Wohlleben, G., C. Trujillo, J. Muller, et al. 2004. Helminth infection modulates the development of allergen-induced airway inflammation. Int. Immunol. 16: 585–596
- Mangan, N. E., R. E. Fallon, P. Smith, et al. 2004. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173: 6346–6356
- Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164: 6406–6416
- Wynn, T. A., A. W. Cheever, M. E. Williams, et al. 1998. IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 160: 4473–4480
- McSorley, H. J., and R. M. Maizels. 2012. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 25: 585–608
- Platts-Mills, T. A., J. A. Woodfolk, E. A. Erwin, and R. Aalberse. 2004. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer. Semin. Immunopathol. 25: 271–279
- Erwin, E. A., K. Wickens, N. J. Custis, et al. 2005. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J. Allergy Clin. Immunol. 115: 74–79
