Abstract
Context: Although autoantibody detection methods such as indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assays (ELISAs) have been available for many years and are still in use the innovation of fast, fully automated instruments using chemiluminescence technology in recent years has led to rapid adoption in autoimmune disease diagnostics. In 2009, BIO-FLASH, a fully automated, random access chemiluminescent analyzer, was introduced, proceeded by the development of the QUANTA Flash chemiluminescent immunoassays (CIA) for autoimmune diagnostics.
Objective: To summarize the evolution of CIAs for the detection of autoantibodies and to review their performance characteristics.
Methods: Pubmed was screened for publications evaluating novel QUANTA Flash assays and how they compare to traditional methods for the detection of autoantibodies. In addition, comparative studies presented at scientific meetings were summarized.
Results: Several studies were identified that compared the novel CIAs with conventional methods for autoantibody detection. The agreements ranged from moderate to excellent depending on the assay. The studies show how the CIA technology has enhanced the analytical and clinical performance characteristics of many autoantibody assays supporting both diagnosis and follow-up testing.
Conclusion: CIA has started to improve the diagnostic testing of autoantibodies as an aid in the diagnosis of a broad range of autoimmune diseases.
Introduction
Over the past 50 years, several important and exciting milestones in the field of immunoassay development have transpired. Latex agglutination assays and radioimmunoassay (RIA) were developed by Singer and PlotzCitation1 in 1956, and Rosalyn Yalow and Solomon BersonCitation2 in 1959, respectively. Several years later, Eva Engvall and Peter Perlman, as well as Bauke van Weemen and Anton Schuurs independently described enzyme-linked immunosorbent assays (ELISA), which represented a significant turning point in immunoassay historyCitation3,Citation4. In 1977, Yalow received the Nobel Prize in Physiology or Medicine for the development of the RIA. In 1983, Anthony Campbell and Ashok PatelCitation5 developed early prototypes of a chemiluminescent immunoassay (CIA). Thereafter, the utility for CIAs grew rapidly, first within the field of clinical chemistry, and then for infectious disease diagnosticsCitation6,Citation7. Inova Diagnostics was among the first diagnostic companies to commercialize CIAs in the field of autoimmunity, notably in the area of celiac disease (CD), anti-phospholipid syndrome (APS), rheumatoid arthritis (RA), small vessel vasculitis (SVV) and connective tissue diseases (CTD). The CIA assays are performed using the BIO-FLASH technology platform, which includes a bench-top, random access analyzer. The benefits of BIO-FLASH are several fold; namely, elimination of sample batching, high reproducibility, sequential results after 30 min, and broad dynamic ranges. Besides the BIO-FLASH system, there are other CIA platforms available for the detection of autoantibodies including the Zenit RA (Menarini Diagnostics, Florence, Italy) or the LIAISON® (Diasorin, Saluggia, Italy). However, based on the broad menu and good assay performance, the BIO-FLASH system is the most widely used CIA in the field of autoimmunity. Consequently, this review article primarily focuses on the technology and studies around QUANTA Flash assays performed on the BIO-FLASH system.
BIO-FLASH instrument and the assay cartridge
The BIO-FLASH chemiluminescent analyzer is a bench-top instrument with a small foot-print. The instrument has capacity for:
Up to 20 reagent cartridges kept at 2–8 °C, together with the cartridge design, assures very good on-board analyte stability
Five sample racks can contain up to 30 primary tubes or cups. Samples can be loaded continuously, as racks are released when the tests for their samples are completed. The instrument identifies samples through a bar-coding system.
280 cuvettes and sufficient consumables (triggers and rinse) to make it a truly walk-away system. Cuvettes can be loaded any time.
Calibrators and controls are presented in bar-coded tubes that fit directly into the sample racks simplifying their identification.
The BIO-FLASH throughput is up to 450 results per 8-h shift, delivering the first result in approximately 30 min. The system is fully automated, random access, with STAT capability. BIO-FLASH software offers a touch screen Microsoft Windows-based user interface and can be linked to a laboratory information system (LIS) for bi-directional connectivity as well as to QUANTA Link.
The reagents, comprised of coated beads, assay buffer, tracer and sample diluent, are contained in a specially designed cartridge () allowing for ease of use. Its design, in combination with the refrigerated reagent chamber (2–8 °C) of the instrument, assures long on-board stability of the reagents.
Figure 1. Concept and protocol of the QUANTA Flash assays. Figure panel (a) illustrates the QUANTA Flash assay cartridge design, which is a key feature making the BIO-FLASH easy to use. Panel (b) shows the overview of QUANTA Flash assay scheme. The procedure involves three reactions (capture of the antibody of interest from the sample by the antigen coupled to the beads; recognition with the ABEI-labelled antibody; chemiluminescent measuring) separated by two washing steps. All steps are performed automatically by BIO-FLASH. Panel (c) illustrates the chemical reaction of the light emission. Using an amide linkage (or pseudo-peptide), the antigen (Ag) or antibody (Ab), indicated as an R in the figure, is attached to the aminobutylethylisoluminol (ABEI) molecule separated by a spacer, therefore reducing the quenching effect of the protein. In the presence of H2O2, a catalyst and high pH, light is emitted. The height and width of the peak of light depends upon the quantity of ABEI captured by the bead, which is directly proportional to the concentration of relevant analyte present in the patient sample. The output is measured over 3 s (from second 9 to second 12) and yields a number value in relative light units (RLUs). In (d), an example of Master (dotted) and Working (green solid) curve is illustrated above. The RLUs are expressed on the y-axis and chemiluminescent units (CU) on the x-axis.
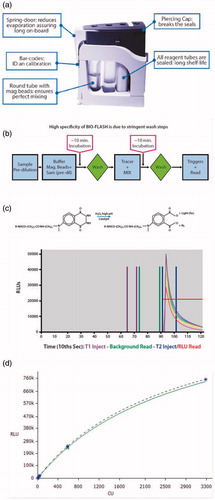
Overview of QUANTA Flash assay principle
The CIAs are designed for the fully automated instrument, containing a luminometer, as well as all the hardware and liquid handling capabilities necessary to perform the assay. The principle and protocol of QUANTA Flash assays are described in several manuscriptsCitation8–10 and are briefly outlined in . Patient samples are pre-diluted by BIO-FLASH with sample buffer in a small disposable plastic cuvette. The system is designed to accommodate primary sample tubes between 12 mm and 16 mm in diameter. Special tubes are also available to accommodate low volume samples. Small amounts of the diluted patient sample, the beads, and the assay buffer are all combined into a second cuvette, mixed, and then incubated for 9.5 min at 37 °C. The paramagnetic beads are sedimented and washed several times. Then, the isoluminol-conjugated antibody is added and the cuvette is incubated for an additional 9.5 min at 37 °C. After the final washing step, the instrument transports the cuvette containing the beads to the luminometer where the triggers (oxidizer and catalyst) are injected. In the luminometer, the light emitting molecule (aminobutylethylisoluminol or ABEI, a luminol derivative) contained in the tracer reagent, is exposed to high pH, an oxidizer (H2O2) and a catalyst. The ABEI-labelled conjugate captured by the beads produces a flash of light as illustrated in , which is measured as a numeric value expressed as relative light units (RLUs). The RLUs are proportional to the amount of isoluminol conjugate that is bound to the antibody, which is in turn proportional to the amount of autoantibodies bound to the target antigen covalently coupled to the beads. The RLUs are translated by the instrument into analyte concentration (e.g. chemiluminescent units or CUs) via a 4-parameter logistic calibration curve (4PLC). The very broad analytical range of QUANTA Flash assays requires the creation of a calibration curve with several points so that its shape is well defined. The 4PLC curve for each lot is established during the manufacturing process of the reagents (beads) and the so-called master curve (MC) is embedded in a bar-code on the test cartridge. Therefore, every batch of QUANTA Flash reagent comes pre-calibrated. The four parameters of this MC are acquired by the instrument via the bar-code of the cartridge. The user has to adapt this MC to the instrument in use, by running two calibrators once per lot. The instrument then re-calculates the 4PLC and uses this new curve, the Working Curve, to extrapolate the results ().
The solid phase: paramagnetic beads
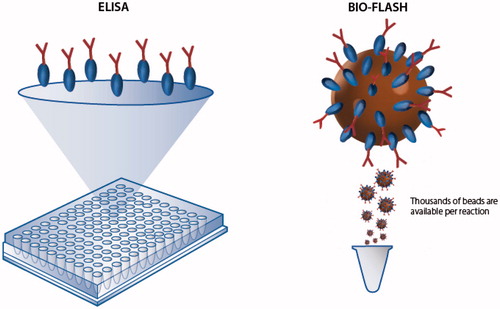
Paramagnetic beads were selected because they offer the possibility of separation steps, which is a requirement in heterogeneous immunoassaysCitation11. Most importantly, paramagnetic bead suspensions behave as a fluid thereby simplifying automation and, unlike with microtiter plates, the tests are performed in a random access mode. Furthermore, bead suspensions present larger surface binding area, a feature that dramatically improves assay sensitivity (). Several protocols can be used to bind either antigen or antibodies to the surface of the paramagnetic particles. This flexibility allows for the development of highly optimized protocols for the detection of autoantibodies.
Figure 2. Illustration of the high surface capacity of paramagnetic beads in comparison to conventional ELISA. Paramagnetic beads provide a significantly higher surface area compared to ELISA plates and are able to bind more antigen or antibody which often results in high analytical sensitivity and a broad analytical measuring range.
Assay characteristics and studies on chemiluminescent immunoassays
Analysis based on chemiluminescence offers excellent sensitivity because of the low background and unequalled broad dynamic range, two or three orders of magnitude wider than any other immunoassayCitation12. A summary of QUANTA Flash assays and their characteristics are found in . Despite the novelty of most QUANTA Flash assays, several studies have already been carried out and reported good assay performanceCitation8–10,Citation12–29.
Table 1. Overview of QUANTA flash assays.
For the anti-extractable nuclear antigen (ENA) family of CIAs, a good agreement between the screening and the confirmatory tests was observed when the QUANTA Flash ENA7 assay was compared to the individual confirmation assays including RNP, Sm, SS-A/Ro60, Ro52, SS-B/La, Scl-70 and Jo-1Citation8. Additionally, good clinical performance for the diagnosis of CTDs has been shown for the anti-ENA QUANTA Flash assays (RNP, Sm, SS-A/Ro60, Ro52, SS-B/La, Scl-70 and Jo-1)Citation8,Citation16.
One very interesting novel assay for the detection of autoantibodies to anti-nuclear antibody (ANA)-associated rheumatic diseases (AARD), the QUANTA Flash CTD Screen Plus, was CE marked in July 2014. The strategy for the development of this complex assay was to include a comprehensive mixture of the most important target autoantigens for the diagnosis of AARD. As a result, 17 highly purified antigens are now part of this novel screening assay. All clinical evaluations on this novel assay showed excellent results. In one of the studies, the QUANTA Flash CTD Screen Plus was compared to the indirect immunofluorescence (IIF) on HEp-2 cellsCitation14. The CIA showed very high specificity for AARD whereas the IIF showed higher sensitivity. However, when both assays were compared at the same specificity (95.1%), the CIA showed a much higher sensitivity (76.4% versus 68.5%). Some studies analyzed the utility of anti-DFS70 antibodies which have been reported primarily in healthy individuals with a positive ANA test, but no evidence of AARD. Therefore, anti-DFS70 antibodies represent a promising tool to improve the diagnostic algorithm of ANA testingCitation9,Citation15,Citation22.
Two recent studies investigated the novel BIO-FLASH assays for the detection of anti-CCP and anti-dsDNA antibodiesCitation27,Citation30. The new QUANTA Flash CCP3 assay showed good performance characteristics in comparison to other assays for the detection of anti-CCP antibodiesCitation27, where the kappa agreements between the QUANTA Flash CCP3 and other methods were excellent (≥0.85). In a comparative study of five assays (Crithidia luciliae IIF test, two commercial dsDNA ELISAs, BioPlex 2200 dsDNA, and QUANTA Flash dsDNA) for the measurement of anti-dsDNA antibodies, QUANTA Flash dsDNA showed the best performance as expressed by the high odds ratio for diagnosing systemic lupus erythematosus (SLE)Citation30.
In a recent study in CD, the QUANTA Flash CD assays were analyzed and compared to another test system for the detection of CD-specific antibodiesCitation12. Although the two systems showed good general agreement and similar performance characteristics for tTG IgA, DGP IgG, and tTG IgG, a significant difference was found for DGP IgA and in the prevalence of CD sera tested with greater than 10 times the upper limit of normal (ULN) for tTG IgACitation12. This difference is of high interest since recently it was proposed that a test result of >10 × ULN represents a strong indicator with high likelihood for CD and alleviates the need for small bowel biopsies in these patientsCitation31.
Although efforts are still ongoing to standardize solid phase methods that detect autoantibodies related to APS, several studies have highlighted the performance of the new QUANTA Flash aCL and β2GPI assays because of their improved analytical performance characteristics and good correlation with the clinical disease status of APS patientsCitation32–34. Additionally, studies have demonstrated the utility of QUANTA Flash β2GPI Domain 1 for the diagnosis of APS as well as its utility in assessment of disease risk in patients being evaluated for APS due to its correlation with APS-related clinical manifestationsCitation10,Citation23,Citation35,Citation36.
Several studies evaluated the performance of chemiluminescent immunoassays for the detection of anti-PR3, anti-MPO, and anti-GBM antibodies for the diagnosis of SVV and Goodpasture’s diseaseCitation25,Citation29,Citation37–39. The kappa agreements between the QUANTA Flash assays and other methods were excellent (>0.8). Three recent studies demonstrated the clinical utility of anti-PR3 antibodies measured by the QUANTA Flash in diseases other than SVV. In two of the studies, anti-PR3 antibodies were able to differentiate ulcerative colitis from Crohn’s diseaseCitation13,Citation20. In the other study, anti-PR3 antibodies were found in high frequency in patients with primary sclerosing cholangitis (PSC)Citation26.
The application of chemiluminescence technology in autoimmunity offers a sensitive and reliable platform for detection of new biomarkers and has facilitated research efforts to develop immunoassays for several important biomarkers in CTD, in particular anti-Th/To antibodies to aid in the diagnosis of systemic sclerosis (SSc)Citation17,Citation21 and anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibodies to aid in the diagnosis of immune-mediated necrotizing myopathies (IMNM)Citation24.
Although the agreement between most of the QUANTA Flash assays and other tests for the detection of autoantibodies is good, some comparison studies and evaluations show low to moderate agreements. This is consistent with the prevailing lack of standardization of certain autoantibody assaysCitation40. The underlying reasons for the discrepancies are manifold and include differences in immobilization chemistries, antigen concentrations, solid phase matrices, sample dilutions, conjugates (secondary antibodies) or washing conditions. In general, it is very difficult to resolve the discrepant findings in comparative studies, in other words, to conclusively prove which immunoassay provides the correct answer. Some autoantibodies can be present in multiple diseases, which make the results difficult to interpret. An important aspect which further complicates the interpretation of comparative studies is the presence of autoantibodies in the pre-clinical phase of many autoimmune diseasesCitation27,Citation28,Citation41,Citation42, a feature which is often erroneously regarded as a “false-positive” test resultCitation43.
When designing comparison studies, it is of utmost importance to avoid sample selection bias. Since some autoantibodies are rare, many laboratories collect and store positive controls over a long period of time and run the samples together with negative samples. For this sample selection, the autoantibody test applied in the routine laboratory is used and the results are then compared to a new technology. Furthermore, there are no published systematic studies that show that the frequency of autoantibodies today is identical to the frequencies observed two or three decades ago. Unpublished anecdotal evidence indicates that some autoantibodies that were at one time seen commonly are now exceedingly rare.
For the standardization of assays, it is important to understand the agreement between different immunoassays. Some immunoassays such as those that detect anti-dsDNA antibodies are known for their low to moderate agreement between methodsCitation44. In contrast, methods for the detection of anti-SS-B/La antibodies often provide excellent agreement between assaysCitation16,Citation45.
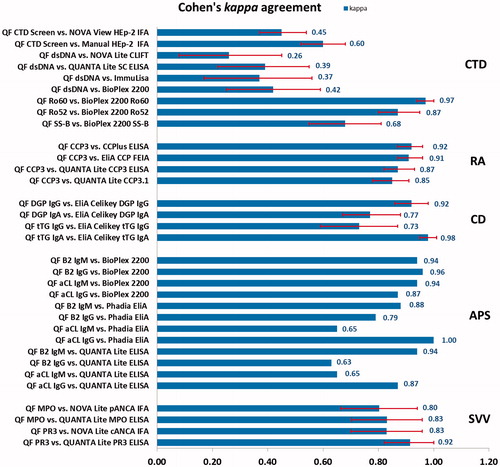
Correlations between methods were analyzed in many studies using Cohen’s kappa agreement test, where moderate agreement corresponds to kappa values between 0.41 and 0.6, substantial agreement corresponds to kappa values between 0.61 and 0.80, and almost perfect agreement corresponds to kappa values of 0.81 or greaterCitation46. Agreement according to Cohen’s kappa between QUANTA Flash assays and other autoantibody detection methods are summarized in , demonstrating a diverse range of qualitative agreement. In conclusion, CIA technology, which has been used in the field of clinical chemistry for many years, now is attaining significant adoption in autoantibody detection.
Figure 3. Agreement between QUANTA Flash assays and other autoantibody detection methods according to Cohen’s kappa agreement test. Red error bars indicate 95% confidence intervals (CI), although not available for all published studies. QF, QUANTA Flash; CTD, connective tissue disease; RA, rheumatoid arthritis; CD, celiac disease; APS, anti-phospholipid syndrome; SVV, small vessel vasculitis.
Acknowledgements
We thank Andrea Seaman for help with final editing of the article.
Declaration of Interest
Michael Mahler and Chelsea Bentow are employed at Inova Diagnostics and Josep Serra at Biokit R&D, companies that manufacture and market autoantibody assays. Marvin Fritzler is a consultant for Inova Diagnostics and has received gifts in kind for research studies. The authors declare no conflicts of interests.
References
- Plotz C, Singer J. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am J Med 1956;21:888–892
- Yalow RS, Berson SA. Assay of plasma insulin in human subjects by immunological methods. Nature 1959;184:1648–1649
- Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971;8:871–874
- Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett 1971;15:232–236
- Campbell AK, Patel A. A homogeneous immunoassay for cyclic nucleotides based on chemiluminescence energy transfer. Biochem J 1983;216:185–194
- Rongen HA, Hoetelmans RM, Bult A, van Bennekom WP. Chemiluminescence and immunoassays. J Pharm Biomed Anal 1994;12:433–462
- Dodeigne C, Thunus L, Lejeune R. Chemiluminescence as diagnostic tool. A review. Talanta 2000;51:415–439
- Bentow C, Swart A, Wu J, et al. Clinical performance evaluation of a novel rapid response chemiluminescent immunoassay for the detection of autoantibodies to extractable nuclear antigens. Clin Chim Acta 2013;424:141–147
- Mahler M, Parker T, Peebles CL, et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol 2012;39:2104–2110
- Mahler M, Norman GL, Meroni PL, Khamashta M. Autoantibodies to domain 1 of beta 2 glycoprotein 1: a promising candidate biomarker for risk management in antiphospholipid syndrome. Autoimmun Rev 2012;12:313–317
- Thompson JA, Bau HH. Microfluidic, bead-based assay: theory and experiments. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:228–236
- Lakos G, Norman GL, Mahler M, et al. Analytical and clinical comparison of two fully automated immunoassay systems for the diagnosis of celiac disease. J Immunol Res 2014;2014:371263
- Arias-Loste MT, Bonilla G, Moraleja I, et al. Presence of anti-proteinase 3 antineutrophil cytoplasmic antibodies (anti-PR3 ANCA) as serologic markers in inflammatory bowel disease. Clin Rev Allergy Immunol 2013;45:109–116
- Bentow C, Lakos G, Rosenblum R, et al. Clinical performance evaluation of a novel, automated chemiluminescent immunoassay, QUANTA Flash CTD Screen Plus. Immunol Res 2015; 61:110–116
- Fitch-Rogalsky C, Steber W, Mahler M, et al. Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. PLoS One 2014;9:e93812
- Infantino M, Bentow C, Seaman A, et al. Highlights on novel technologies for the detection of antibodies to Ro60, Ro52, and SS-B. Clin Dev Immunol 2013;2013:978202
- Mahler M, Satoh M, Hudson M, et al. Autoantibodies to the Rpp25 component of the Th/To complex are the most common antibodies in patients with systemic sclerosis without antibodies detectable by widely available commercial tests. J Rheumatol 2014;41:1334–1343
- Mahler M, Meroni PL, Bossuyt X, Fritzler MJ. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J Immunol Res 2014;2014:315179
- Mahler M, Dervieux T. Comments on recent advances and recommendations for the assessment of autoantibodies to cellular antigens referred as antinuclear antibodies. Ann Rheum Dis 2014;73:e36
- Mahler M, Bogdanos DP, Pavlidis P, et al. PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease. Clin Chim Acta 2013;424:267–273
- Mahler M, Gascon C, Patel S, et al. Rpp25 is a major target of autoantibodies to the Th/To complex as measured by a novel chemiluminescent assay. Arthritis Res Ther 2013;15:R50
- Miyara M, Albesa R, Charuel JL, et al. Clinical phenotypes of patients with anti-DFS70/LEDGF antibodies in a routine ANA referral cohort. Clin Dev Immunol 2013;2013:703759
- Mondejar R, Gonzalez-Rodriguez C, Toyos-Saenz de Miera FJ, et al. Role of antiphospholipid score and anti-beta2-glycoprotein I Domain I autoantibodies in the diagnosis of antiphospholipid syndrome. Clin Chim Acta 2014;431:174–178
- Musset L, Miyara M, Benveniste O, et al. Analysis of autoantibodies to 3-hydroxy-3-methylglutaryl-coenzyme A reductase using different technologies. J Immunol Res 2014;2014:405956
- Noel N, Andre C, Bengoufa D, et al. Performance evaluation of three assays for the detection of PR3-ANCA in granulomatosis with polyangiitis in daily practice. Autoimmun Rev 2013;12:1118–1122
- Stinton LM, Bentow C, Mahler M, et al. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC). PLoS One 2014;9:e112877
- Webb T, Lakos G, Swart A, et al. Clinical evaluation of a novel chemiluminescent immunoassay for the detection of anti-citrullinated peptide antibodies. Clin Chim Acta 2014;437:161–167
- Yee A, Webb T, Seaman A, et al. Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol Res 2015;61:24–30
- Mahler M, Radice A, Sinico RA, et al. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: an international multicenter study. Nephrol Dial Transplant 2012;27:243–252
- Infantino M, Meacci F, Bentow C, et al. Clinical comparison of QUANTA flash dsDNA chemiluminescent immunoassay with four current assays for the detection of anti-dsDNA autoantibodies. J Immunol Res 2015;2015:902821
- Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160
- Van HF, Persijn L, Decavele AS, Devreese K. Performance of two new, automated chemiluminescence assay panels for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int J Lab Hematol 2012;34:630–640
- Noubouossie D, Valsamis J, Corazza F, et al. An automated chemiluminescence immunoassay may detect mostly relevant IgG anticardiolipin antibodies according to revised Sydney criteria. Acta Clin Belg 2012;67:184–189
- Forastiero R, Papalardo E, Watkins M, et al. Evaluation of different immunoassays for the detection of antiphospholipid antibodies: report of a wet workshop during the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta 2014;428:99–105
- Ciesla M, Wypasek E, Undas A. IgA antiphospholipid antibodies and anti-domain 1 of beta 2 glycoprotein 1 antibodies are associated with livedo reticularis and heart valve disease in antiphospholipid syndrome. Adv Clin Exp Med 2014;23:729–733
- Pengo V, Ruffatti A, Tonello M, et al. Antiphospholipid syndrome: antibodies to domain 1 of beta2-glycoprotein 1 correctly classify patients at risk. J Thromb Haemost 2015;13:782–787
- Schulte-Pelkum J, Radice A, Norman GL, et al. Novel clinical and diagnostic aspects of antineutrophil cytoplasmic antibodies. J Immunol Res 2014;2014:185416
- Mahler M, Radice A, Yang W, et al. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin Chim Acta 2012;413:719–726
- Hirose O, Itabashi M, Takei T, Nitta K. Comparison of a novel chemiluminescence enzyme immunoassay (CLEIA) with enzyme-linked immunosorbent assay (ELISA) for the determination of MPO-ANCA in patients with ANCA-associated vasculitis. Mod Rheumatol 2015;25:230–234
- Chan EK, Fritzler MJ, Wiik A, et al. AutoAbSC.Org – autoantibody standardization committee in 2006. Autoimmun Rev 2007;6:577–580
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–1533
- Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev 2012;12:318–322
- Fritzler MJ. Toward a new autoantibody diagnostic orthodoxy: understanding the bad, good and indifferent. Autoimmun Highlight 2012;3:51–58
- Mahler M, Fritzler MJ. Anti-dsDNA antibody testing in the clinic: farr or ELISA? Nat Clin Pract Rheumatol 2007;3:72–73
- Hanly JG, Su L, Farewell V, Fritzler MJ. Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Method 2010;358:75–80
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360–363
- Infantino M, Meacci F, Bentow C, et al. Clinical comparison of QUANTA Flash dsDNA chemiluminescent immunoassay with four current assays for the detection of anti-dsDNA autoantibodies. J Immunol Res 2015;2015:902821
