Abstract
Context: Smoking is associated with increased fibrinogen and decreased paraoxonase (PON) activity, markers of inflammation and oxidative stress, in patients with coronary artery disease.
Objective: We tested the hypothesis that the adverse effect of smoking on these biomarkers of inflammation and oxidative stress would be detectable in otherwise healthy young female habitual smokers.
Materials and methods: Thirty-eight young women participated in the study (n = 20 habitual smokers, n = 18 non-smokers). Fibrinogen, PON-1 activity and HDL oxidant index (HOI) were measured.
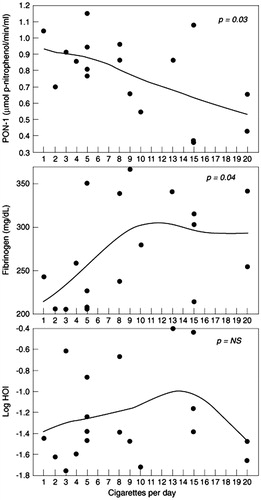
Results: Mean values of fibrinogen, PON-1 activity and log HOI were not different between the groups. Importantly, however, decreased PON-1 activity (rs = −0.51, p = 0.03) and increased fibrinogen (rs = 0.49, p = 0.04) were significantly correlated with increasing number of cigarettes smoked per day in habitual smokers.
Discussion and conclusion: Cigarette smoking is associated with a dose-dependent adverse effect on PON-1 activity and fibrinogen in young women, which may have implications for future cardiovascular risk.
Introduction
Cardiovascular disease is the number one cause of death in women, but is understudied in this population. The onset of cardiovascular disease in women lags that of men by approximately 10 years (Worrall-Carter et al., Citation2011). In fact, pre-menopausal women rarely suffer from acute coronary syndromes, except if they smoke cigarettes. Cigarette smoking virtually eliminates the gender advantage, increasing cardiovascular risk in young menstruating females by 7-fold (Njolstad et al., Citation1996). In fact, smoking confers a greater risk of premature first myocardial infarction in women compared to men (Bahler et al., Citation2012). We have recently demonstrated that pre-menopausal habitual smokers have increased sympathetic nerve activity (SNA) compared to non-smokers, mediated by blunted baroreflex control (Middlekauff et al., Citation2013; Park & Middlekauff, Citation2009). Importantly, increased SNA and attenuated baroreflexes have been shown to increase cardiovascular risk in several populations (Barretto et al., Citation2009; Farrell et al., Citation1992; La Rovere et al., Citation1998). Increased oxidative stress and inflammation, induced by cigarette smoke exposure, also increase risk for atherosclerotic disease and acute coronary syndromes (Ambrose & Barua, Citation2004; Csordas & Bernhard, Citation2013). It is unknown whether, like SNA, increased oxidative stress and inflammation are present in young female smokers, thereby potentially contributing to the initiation and premature presentation of coronary atherosclerotic disease. Accordingly, we tested the hypothesis that paraoxonase (PON-1) activity (James et al., Citation2000) and high-density lipoprotein (HDL) anti-oxidant capacity, determined by a HDL oxidative index (HOI) (Breton et al., Citation2014), which confer protection against oxidative stress in blood would be perturbed by cigarette smoking, and that fibrinogen, a marker of inflammation (Kaptoge et al., Citation2012), would be elevated in otherwise healthy young female smokers.
Methods
Study population
Volunteers in this study overlap with those in our recent report (Middlekauff et al., Citation2013). A total of 38 healthy pre-menopausal women including 20 non-smokers and 18 habitual cigarette smokers participated. All were between 18 and 45 years, with no chronic medical problems, were not taking medications including contraceptives or nicotine gum/patches, and did not use illicit drugs or drink ≥2 alcoholic drinks per day. No volunteers participated in a regular exercise training program. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each volunteer. On the day of the study, a negative urine pregnancy test (Pregnancy Test strip, Early-Pregnancy-Test.com) was confirmed.
After abstaining from caffeine and tobacco for at least 12 h, volunteers were placed in a supine position in a quiet, semi-dark, temperature-controlled (21 °C) Human Physiology Laboratory located in the UCLA General Clinical Research Center and venopuncture was performed. Blood was drawn into pre-iced heparinized vacutainers, and placed on ice. One tube of blood was sent immediately to the UCLA Clinical Laboratory for standard fibrinogen assay. For fibrinogen, the within assay coefficient of variation (CV) is 0.95%, and the inter-assay CV is 1.1%. Another tube was centrifuged to separate plasma for analyses of anti-oxidant parameters (HOI and PON-1 activity); plasma samples were frozen at −80 °C in a cryopreservative solution as previously described (Breton et al., Citation2014).
Paraoxonase-1 assay
The paraoxonase family of enzymes exists in three isoforms, paraoxonase-1, -2 and -3 (PON-1, -2 and -3). PON-1 is the only isoform with the ability to hydrolyze organophosphates such as paraoxon (Aldridge, Citation1953a,Citationb). In this assay, we determined that the ability of plasma PON-1 which is mainly associated with HDL, to hydrolyze paraoxon substrate. The hydrolysis of paraoxon (diethyl-p-nitrophenyl phosphate) to p-nitrophenol by PON-1 was determined by incubating 5 µl of plasma with 1.0 mM paraoxon in 100 mM tris–HCl buffer (pH 8.5), as previously described (Yin et al., Citation2013). The kinetics of p-nitrophenol formation was determined by recording absorbance at 405 nm every 15 s for 4 min. The enzyme activity was expressed as µmol p-nitrophenol formed per minute for every 1 ml of plasma. The samples were assayed in triplicates. The intra-assay CV for the assay was 2.6% and the inter-assay CV was 8.95%.
Total and HDL cholesterol (HDL-c)
The LipiDirect Magnetic HDL reagent (Reference Diagnostics, Inc., Bedford, MA) is a magnetically enhanced reagent containing dextran sulfate (MW 50 000) and magnesium chloride which allows quick isolation of total HDL cholesterol by selectively precipitating low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL). After a single centrifugation step, only HDL-c remains in the supernatant. HDL-c was isolated by incubating 50 µl of plasma with 10 µl of LipiDirect Magnetic HDL cholesterol precipitating reagent (Reference Diagnostics, Inc., Bedford, MA) for 10 min at room temperature. The mixture was centrifuged at 12 000rpm for 5 min at 4 °C and the HDL containing supernatant was collected. Total and HDL cholesterol concentrations were determined using the Infinity Cholesterol Reagent and cholesterol standard (Thermo Scientific, Middletown, VA).
HDL anti-oxidant assay
HDL anti-oxidant capacity was determined as the ability of HDL to inhibit LDL-induced oxidation of dihydrodichlorofluorescein (DCFH) into the fluorescent dichlorofluorescein (DCF). The assay was performed as previously described (Sarkar et al., Citation2006; Yin et al., Citation2013). Briefly, HDL cholesterol was isolated from plasma as described above. The DCFH reagent was prepared by incubating dichlorofluorescein diacetate (DCF-DA) (Sigma-Aldrich, St. Louis, MO) at 2 mg/ml with 0.1 M NaOH. DCF-DA was hydrolyzed to DCFH and the mixture was neutralized with 10× PBS. Equal concentrations of HDL and LDL cholesterol (50 µg/ml) were mixed with Tris-HCl buffer (pH 7.4) up to a total volume of 100 µl and added to a black well flat bottom microtiter plate in triplicate. The plate was incubated at 37 °C for 1 h. Next, 20 µl of the DCFH reagent was added to each well and the plate was incubated at 37 °C for 3 h. DCF fluorescence intensity was determined using a plate reader (SynergyMx, BioTek, Winooski, VT) at an excitation wavelength of 485 nm and emission wavelength of 530 nm. HDL anti-oxidant capacity was expressed as a HOI, determined by the ratio of DCF fluorescence in the presence and absence of HDL. An index <1.0 denotes protective anti-oxidant HDL whereas an index > 1.0 indicates pro-oxidant HDL. The variability of the assay has been previously reported (Breton et al., Citation2014). The samples were assayed in triplicates. The within assay CV was 6.89%. The inter-assay CV for 4 separate measurements over a period of two months was 7.30%.
Statistical analysis
Normal quantile–quantile plots of PON-1, fibrinogen and HOI were used to assess whether these variables follow the normal distribution; PON-1 activity, fibrinogen and log HOI follow the normal distribution. Therefore, for HOI only, mean comparisons were carried out on the log scale. Means were compared between the groups using t tests. Covariate adjusted means were computed under a regression analysis of covariance model adjusting for the four covariates: age, BMI, HDL-c and total cholesterol. Under the adjustment model, linear and nonlinear (quadratic) effects of each of these four covariates on an outcome were allowed along with the interaction of each of the four covariates with group. Correlations between two continuous variables were assessed by computing the nonparametric Spearman correlation coefficient (rs). Spine and linear regression was used to assess simultaneously the effects of pack years, cigarettes per day and smoking duration on PON-1, fibrinogen and log HOI in the habitual smokers. Values of p < 0.05 were considered statistically significant.
Results
Descriptive characteristics
Characteristics, including age and body mass index are displayed in and did not differ between the groups. Habitual smokers smoked an average of 9.1 ± 1.4 cigarettes per day (self-reported) for a mean duration of 8.8 ± 1.5 years.
Table 1. Study population characteristics.
PON-1 activity, fibrinogen and log HOI
The mean PON-1 activity, fibrinogen and log HOI unadjusted or adjusted by age, BMI, HDL cholesterol and total cholesterol, did not show significant differences between smokers and non-smokers in this study population (). However, assessment of correlation between these functional variables and the number of cigarettes smoked per day in the smoker group, showed significant association with PON-1 activity (, top) and fibrinogen (, middle) but not with log HOI (, bottom). PON-1 activity was significantly, and negatively, associated with the number of cigarettes smoked per day (rs = −0.51, p = 0.03) while fibrinogen significantly increased with increasing number of cigarettes smoked per day (rs = 0.49, p = 0.04).
Discussion
Cigarette smoke exposure increases oxidative stress and inflammation, leading to vascular dysfunction that can lead to the premature onset of cardiovascular disease (Ambrose & Barua, Citation2004). The novel finding of the present study is that PON-1 activity is decreased and fibrinogen is increased in a dose-dependent manner in otherwise healthy young female smokers, consistent with the concept that these adverse processes are activated early, well before atherosclerosis would be expected to be clinically apparent. PON-1 is an ester hydrolase enzyme associated with HDL in blood and prevents the formation of oxidized LDL, thus defending against key early steps in the initiation and evolution of atherosclerosis (Watson et al., Citation1995). Interestingly, decreased PON-1 activity was found to be an independent predictor, along with abnormal lipid profile, of premature coronary artery disease in patients younger than 45 years, (mean age 40.7 years) (Sarkar et al., Citation2006). PON-1 activity varies substantially among humans, influenced by diet and activity level independent of PON-1 polymorphisms; patients with hyperlipidemia and diabetes have lower PON-1 activity than controls (Mackness et al., Citation1993; Senti et al., Citation2003). Comparing only mean values of PON-1 activity, which is influenced by several environmental influences, may obscure important associations. PON-1 has been shown to be decreased in some (Haj Mouhamed et al., Citation2012; Solak et al., Citation2005), but not all (Aslan et al., Citation2014), studies of older smokers compared to non-smokers. For example, in middle-aged heavy smokers, but not mild or moderate smokers, PON activity was found to be significantly decreased compared to non-smokers (Solak et al., Citation2005). In patients with angiographically-proven coronary artery disease, smoking is correlated with decreased PON-1 activity, and lower PON-1 activity is associated with the severity of coronary artery disease (James et al., Citation2000). Our study extends the association of smoking with decreased PON-1 activity to young (mean age 26 years) female smokers without known coronary artery disease, in whom a dose-dependent reduction of PON-1 activity is related to increased number of cigarettes smoked per day. This evidence of smoking-related oxidative stress, a known mechanism by which smoking promotes premature atherosclerosis, coupled with our previous findings of increased sympathetic nerve activity and blunted baroreflex control in this otherwise healthy female population (Middlekauff et al., Citation2013), mandates redoubled efforts to encourage smoking cessation in the young, especially young women.
Fibrinogen, an acute phase reactant and a critical component of thrombosis, is of value in predicting first cardiovascular events (Kaptoge et al., Citation2012). Plasma fibrinogen levels are significantly higher in healthy young male smokers when compared to non-smokers and smoking is independently associated with plasma fibrinogen levels in a random sample of healthy young men (Cigolini et al., Citation1994; Dotevall et al., Citation1987). Further, in a study with gender-matched volunteers ranging from 20 to 60 years, a positive correlation between smoking intensity and plasma fibrinogen levels has been found (Fernandez et al., Citation2002). In fact, reduction in the mean number of cigarettes smoked per day results in significant improvement in fibrinogen levels in healthy smokers (Eliasson et al., Citation2001). Our study extends these results to a healthy, pre-menopausal female cohort, in whom we found a statistically significant dose-dependent association between increasing number of cigarettes smoked with increasing fibrinogen levels.
Plasma HDL levels may be inversely correlated with atherosclerosis and cardiac risk, although the relationship is complex (Ansell et al., Citation2003; Navab et al., Citation2001). One mechanism by which HDL may be protective is through its anti-oxidant and anti-inflammatory properties, which could be modulated in subjects with coronary artery disease. For instance, in patients with unstable compared with stable coronary artery disease, a decrease in the anti-oxidant capacity of HDL has been found (Patel et al., Citation2011). Cigarette smoking has been reported to promote oxidation in plasma lipoproteins and alter HDL functionality (Ueyama et al., Citation1998). We tested whether smoking could have altered HOI, which we have reported to be quite sensitive to the exposure of environmental pollutants such as diesel exhaust (Yin et al., Citation2013) and ambient ultrafine particles (Li et al., Citation2013). In the present study, increasing smoking intensity did not correlate with increasing HOI, as it did with decreasing PON-1 and increasing fibrinogen. It is possible that other factors, in addition to PON-1 activity, must be perturbed to result in HDL anti-oxidant dysfunction. This is consistent with our findings that while ApoE null mice exposed to diesel exhaust for two weeks, exhibited decreased PON-1 activity and HDL anti-oxidant dysfunction, the latter remained perturbed, even after full restoration of PON-1 activity that was induced by the breathing of clean filtered air for an additional week (Yin et al., Citation2013). Potential gender-specific differences in HDL function could explain the lack of association of HDL anti-oxidant function with smoking intensity in our study. Estrogen increases HDL levels and alters its vascular and anti-oxidant activities, thereby potentially protecting pre-menopausal women from cardiovascular disease (Abplanalp et al., Citation2000; Gong et al., Citation2003; Hockerstedt et al., Citation2004).
Conclusions
In summary, in this study we address the critical issue of potential mechanisms underlying the premature cardiac risk in young smokers, a risk which is even greater in young female compared to male smokers. We report the novel finding that cigarette smoking in otherwise healthy young women is associated with a dose-dependent adverse effect on PON-1 activity and fibrinogen, consistent with the presence of pro-oxidative and inflammatory effects of smoking in the absence of overt atherosclerosis. In an earlier report (Middlekauff et al., Citation2013) we found that young female smokers compared to non-smokers had an increased sympathetic burden and decreased baroreflex function, both known to be associated with increased cardiac risk. These novel findings of early adverse smoking effects in asymptomatic young women must be used to encourage young women to quit smoking before the onset of clinically manifest coronary artery disease, and should be investigated in young male smokers as well.
Declaration of interest
This research is supported by funds from the Tobacco-Related Disease Research Program Exploratory/Developmental Research Award 18XT-00115 (to HRM), the Clinical & Translational Science Institute Grant #UL1TR000124, and the National Institute of Environmental Health Sciences RO1 Award ES016959 (to JAA). No author reports a conflict of interest.
Supplementary Material
Download PDF (119.1 KB)Supplementary Material
Download PDF (1.2 MB)Supplementary Material
Download PDF (1.2 MB)Supplementary Material
Download PDF (1.2 MB)Supplementary Material
Download PDF (1.2 MB)Supplementary Material
Download PDF (1.2 MB)References
- Abplanalp W, Scheiber MD, Moon K, et al. (2000). Evidence for the role of high density lipoproteins in mediating the antioxidant effect of estrogens. Eur J Endocrinol 142:79–83
- Aldridge WN. (1953a). Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J 53:110–17
- Aldridge WN. (1953b). Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J 53:117–24
- Ambrose JA, Barua RS. (2004). The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43:1731–7
- Ansell BJ, Navab M, Hama S, et al. (2003). Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108:2751–6
- Aslan R, Kutlu R, Civi S, Tasyurek K. (2014). The correlation of the total antioxidant status (TAS), total oxidant status (TOS) and paraoxonase activity (PON1) with smoking. Clin Biochem 47:393–7
- Bahler C, Gutzwiller F, Erne P, Radovanovic D. (2012). Lower age at first myocardial infarction in female compared to male smokers. Eur J Prev Cardiol 19:1184–93
- Barretto AC, Santos AC, Munhoz R, et al. (2009). Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135:302–7
- Breton CV, Yin F, Wang X, et al. (2014). HDL anti-oxidant function associates with LDL level in young adults. Atherosclerosis 232:165–0
- Cigolini M, Targher G, DE Sandre G, et al. (1994). Plasma fibrinogen in relation to serum insulin, smoking habits and adipose tissue fatty acids in healthy men. Eur J Clin Invest 24:126–30
- Csordas A, Bernhard D. (2013). The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 10:219–30
- Dotevall A, Kutti J, Teger-Nilsson AC, et al. (1987). Platelet reactivity, fibrinogen and smoking. Eur J Haematol 38:55–9
- Eliasson B, Hjalmarson A, Kruse E, et al. (2001). Effect of smoking reduction and cessation on cardiovascular risk factors. Nicotine Tob Res 3:249–55
- Farrell TG, Odemuyiwa O, Bashir Y, et al. (1992). Prognostic value of baroreflex sensitivity testing after acute myocardial infarction. Br Heart J 67:129–37
- Fernandez JA, Gruber A, Heeb M, Griffin JH. (2002). Protein C pathway impairment in nonsymptomatic cigarette smokers. Blood Cells Mol Dis 29:73–82
- Gong M, Wilson M, Kelly T, et al. (2003). HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J Clin Invest 111:1579–87
- Haj Mouhamed D, Ezzaher A, Mechri A, et al. (2012). Effect of cigarette smoking on paraoxonase 1 activity according to PON1 L55M and PON1 Q192R gene polymorphisms. Environ Health Prev Med 17:316–21
- Hockerstedt A, Jauhiainen M, Tikkanen MJ. (2004). Lecithin/cholesterol acyltransferase induces estradiol esterification in high-density lipoprotein, increasing its antioxidant potential. J Clin Endocrinol Metab 89:5088–93
- James RW, Leviev I, Righetti A. (2000). Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation 101:2252–7
- Kaptoge S, Di Angelantonio E, Pennells L, et al. (2012). C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 367:1310–20
- La Rovere MT, Bigger JT Jr, Marcus FI Jr, et al. (1998). Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351:478–84
- Li R, Navab M, Pakbin P, et al. (2013). Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res 54:1608–15
- Mackness MI, Arrol S, Abbott CA, Durrington PN. (1993). Is paraoxonase related to atherosclerosis. Chem Biol Interact 87:161–71
- Middlekauff HR, Park J, Agrawal H, Gornbein JA. (2013). Abnormal sympathetic nerve activity in women exposed to cigarette smoke: a potential mechanism to explain increased cardiac risk. Am J Physiol Heart Circ Physiol 305:H1560–7
- Navab M, Hama SY, Hough GP, et al. (2001). A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42:1308–17
- Njolstad I, Arnesen E, Lund-Larson PG. (1996). Smoking, serum lipids, blood pressure and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark study. Circulation 93:450–6
- Park J, Middlekauff HR. (2009). Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol 297:H564–8
- Patel PJ, Khera AV, Jafri K, et al. (2011). The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol 58:2068–75
- Sarkar PD, Shivaprakash TM, Madhusudhan B. (2006). Association between paraoxonase activity and lipid levels in patients with premature coronary artery disease. Clin Chim Acta 373:77–81
- Senti M, Tomas M, Anglada R, et al. (2003). Interrelationship of smoking, paraoxonase activity, and leisure time physical activity: a population-based study. Eur J Intern Med 14:178–84
- Solak ZA, Kabaroglu C, Cok G, et al. (2005). Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin Exp Med 5:99–105
- Ueyama K, Yokode M, Arai H, et al. (1998). Cholesterol efflux effect of high density lipoprotein is impaired by whole cigarette smoke extracts through lipid peroxidation. Free Radic Biol Med 24:182–90
- Watson AD, Berliner JA, Hama SY, et al. (1995). Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 96:2882–91
- Worrall-Carter L, Ski C, Scruth E, et al. (2011). Systematic review of cardiovascular disease in women: assessing the risk. Nurs Health Sci 13:529–35
- Yin F, Lawal A, Ricks J, et al. (2013). Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 33:1153–61