Abstract
A non-cancer inhalation chronic toxicity assessment for diethylamine (DEA, CAS number 109-89-7) was conducted by the Texas Commission on Environmental Quality. A chronic Reference Value (ReV) was determined based on a high-quality study conducted in mice and rats by the National Toxicology Program. Chronic inhalation ReVs are health-based exposure concentrations used in assessing health risks of long-term (i.e. lifetime) chemical exposure. DEA is used industrially as an organic intermediate to produce corrosion inhibitors, and is widely used in rubber, pharmaceuticals, resins, pesticides, insect repellants, dye processing and as a polymerization inhibitor. Although systemic effects have been noted at higher concentrations, DEA acts primarily as a respiratory irritant with effects occurring in the upper respiratory tract. Rats were exposed to 0, 31, 62.5 and 125 ppm DEA and mice to 0, 16, 31 and 62.5 ppm DEA for 6 h/day, 5 days/week for 105 weeks. Mice were slightly more sensitive than rats. The critical effect identified in mice was hyperostosis in the turbinates although DEA caused a number of other non-neoplatic lesions. Dose–response data were suitable to benchmark concentration (BMC) modeling. The human equivalent point of departure (PODHEC) was calculated from the 95% lower limit of the BMC(10) using default duration and animal-to-human dosimetric adjustments. Total uncertainty factors of 90 were applied to the PODHEC to account for variation in sensitivity within the human population, toxicodynamic differences between mice and humans, and database uncertainty. The chronic ReV for DEA is 11 ppb (33 µg/m3).
Introduction
Diethylamine (DEA) is used as an organic intermediate in industry to produce the corrosion inhibitor, N,N-diethylethanolamine. It is widely used in rubber, pharmaceuticals, resins, pesticides, insect repellants and dye processing. In addition, DEA may be used as a polymerization inhibitor [American Conference of Governmental Industrial Hygienists (ACGIH), Citation2001; National Toxicology Program (NTP), Citation2011]. DEA has ubiquitous occurrence in trace amounts (NTP, Citation2011). Neurath et al. (Citation1977) and Uhegbu & Maduagwu (Citation1995) reported that DEA occurs naturally in foods and plants. According to Grant (Citation1986), DEA is produced during decay of fish. Information submitted to the U.S. Environmental Protection Agency (USEPA) by companies for chemicals under the 1989–2002 inventory update rule indicates U.S. production of DEA ranged from 10 to 50 million pounds [Hazardous Substance Database (HSDB), Citation2015)] which makes it a high-production volume chemical.
Exposure to DEA can be through inhalation, ingestion or dermal routes. The purpose of this study is to evaluate adverse effects of DEA exposure through the inhalation route. DEA is not monitored for by the Texas Commission on Environmental Quality’s (TCEQ) ambient air monitoring program, so currently no ambient air data (i.e. peaks, annual averages and trends) are available. However, Key et al. (Citation2011) developed an analytical method to measure trace nitrogenous atmospheric bases in ambient air. The concentration of DEA measured in ambient air in Hermon Park (Los Angeles, CA) ranged from non-detected to 28 part per trillion by volume DEA sampled for 3-h periods between 4 and 7 pm over the course of 5 days in September 2009.
DEA is a respiratory irritant whose effects occur primarily in the upper respiratory tract. The acute inhalation toxicity of DEA has been investigated in rodents by a variety of investigators (Lynch et al., Citation1986; NIOSH, Citation1987; NTP, Citation2011; Union Carbide, Citation1950; Virginia Chemicals, Citation1987). Typically, systemic effects were observed at higher concentrations than required to induce respiratory effects. When in contact with the eyes and nose, DEA can lead to sensory irritation in humans (Lundqvist et al., Citation1992; Mackison et al., Citation1981). The TCEQ has developed a 1-h acute reference value (ReV) of 110 ppb (330 µg/m3) based on Lundqvist et al. (Citation1992) with the critical effect of nasal and eye sensory irritation in humans (TCEQ, Citation2015a). An acute ReV is an estimation of an inhalation exposure for the human population (including susceptible subgroups) that is likely to be without an appreciable risk of adverse effects after a one-h exposure.
Chronic toxicity factors have not been developed for DEA even though a high-quality NTP study was made available in 2011. The purpose of this study is to develop a chronic inhalation ReV for DEA based on the NTP (Citation2011) study where rodents were exposed via inhalation to DEA for 14 and 105 weeks. A chronic ReV is an estimation of an inhalation exposure for the human population (including susceptible subgroups) that is likely to be without an appreciable risk of adverse effects for exposure over a lifetime. The derivation of the ReV for DEA is discussed in more detail in the TCEQ Development Support Document, a summary document that describes the steps involved in a toxicity assessment (TCEQ, Citation2015a).
Methods
The TCEQ Guidelines (TCEQ, Citation2015b) employ the 4-step risk assessment process formalized by the National Research Council (NRC, Citation1983, Citation1994) and procedures recommended in numerous USEPA risk assessment guidance documents and the scientific literature (NRC, Citation2001; USEPA, Citation1994, Citation2002) for chemicals with adequate toxicity data. Chronic ReVs are derived using dose–response data and identifying an appropriate point of departure (POD) (e.g. a lowest- and no-observed-adverse-effect level (LOAEL and NOAEL, respectively). Briefly, the steps include: (1) review essential data including physical/chemical properties and select key studies, (2) conduct a mode of action (MOA) analysis, (3) choose the appropriate dose metric, (4) determine the POD for each relevant endpoint, (5) conduct appropriate dosimetric modeling and (6) extrapolate from the adjusted POD to lower exposures based on MOA analysis and select critical effect.
Development of the inhalation reference value
Physical/chemical properties
Diethylamine is a colorless liquid at room temperature with a fishy, ammonia-like odor [ACGIH, Citation2001; National Institute of Occupational Safety and Health (NIOSH), Citation2011]. Since the vapor pressure is 192 mm Hg at 20 °C (NIOSH, Citation2011), DEA is present in ambient air as a vapor. It has a molecular weight of 73.1 g mol−1 and is flammable. DEA is miscible with water. As reported in HSDB (Citation2015), DEA is soluble in alcohol, ether, carbon tetrachloride, chloroform, paraffin hydrocarbons, aromatic and aliphatic hydrocarbons, fixed oils, mineral oils, and oleic and stearic acids. It has a low octanol-water partition coefficient (Kow) of 0.58 (HSDB, Citation2015), which indicates it is unlikely to bioconcentrate. Other physical/chemical properties of DEA may be found in . shows the chemical structure of DEA, a secondary amine.
Figure 1. Chemical structure of DEA. When amines with a high pKa come in contact with tissues or fluids at physiologic pH, they become protonated and hydroxide ion is released, causing local necrosis.
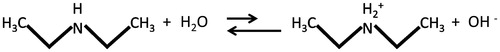
Table 1. Chemical and physical data.
Literature search
NTP (Citation2011) and Montelius (Citation2013) recently reviewed the toxicity of DEA. The TCEQ conducted a review of the toxicity studies in these documents and also conducted a thorough review of the literature since 2008. There are no published epidemiology investigations or reports of chronic health effects in humans with adequate DEA exposure data to develop a chronic ReV. According to NIOSH (Citation2011), short-term inhalation exposure of occupational workers to DEA may cause initial corrosive effects on eyes and/or airways. Lung edema and pneumonitis may then occur. Severe swelling of the throat may occur after exposure at high levels.
Lundqvist et al. (Citation1992) evaluated acute effects in healthy non-smoking subjects after DEA exposure at 25 ppm for 15 min. There were no significant differences in nasal irritation measures and nasal airway volume. Acute sensory effects were evaluated in a group of five healthy, non-smoking adult males exposed to a time-weighted average of 10 ppm of DEA for 60 min. Subjects reported strong olfactory responses and distinct nasal and eye irritation. Since there were no subchronic or chronic human studies, animal studies were used to derive the chronic ReV.
High-quality subchronic and chronic animal studies were conducted by NTP (Citation2011), which produced the lowest NOAEL and LOAEL values when compared with two other subchronic animal studies (Brieger & Hodes, Citation1951; Lynch et al., Citation1986). provides a comparison of pertinent subchronic and chronic inhalation studies. The subchronic 14-week and chronic 105-week NTP studies were conducted in compliance with Food and Drug Administration Good Laboratory Practice Regulations (21 CFR, Part 58). An on-line gas chromatograph was used to monitor chamber and room concentrations of DEA. The following sections focus on the 14- and 105-week NTP (Citation2011) investigations.
Table 2. Chronic and subchronic inhalation studies.
Key studies
F344/N rats and B6C3F1 mice were treated with DEA 6 h/day, 5 days/weeks for 14 or 105 weeks (NTP, Citation2011). Both rats and mice were observed twice daily and were weighed initially and then weekly for the first 13 weeks; clinical findings were recorded every 4 weeks for the first 13 weeks; afterward, body weights and clinical findings were recorded every 4 weeks through week 93; then every 2 weeks, and at the end of the studies. Complete histopathology was performed on all the rats and mice. In addition to gross lesions and tissue masses, the following tissues were examined: adrenal gland, bone with marrow, brain, clitoral gland, esophagus, eyes, gallbladder (mice only), Harderian gland, heart, large intestine (cecum, colon and rectum), small intestine (duodenum, jejunum and ileum), kidney, larynx, liver, lungs, lymph nodes (bronchial, mandibular, mediastinal and mesenteric), mammary gland, nose, ovary, pancreas, parathyroid gland, pituitary gland, preputial gland, prostate gland, salivary gland, skin, spleen, stomach (forestomach and glandular), testis with epididymis and seminal vesicle, thymus, thyroid gland, trachea, urinary bladder, uterus and Zymbal’s gland (rats only). Full hematology and clinical chemistry parameters were evaluated in the 14-week study but not for the 105-week study.
105-week study in rats
Groups of 50 male and 50 female rats were exposed to DEA vapor at concentrations of 0 (control), 31, 62.5 or 125 ppm, 6 h/day, 5 days/week for 105 weeks (NTP, Citation2011). Survival of exposed groups of rats was similar to that of the chamber control groups. Mean body weights of males and females exposed to 125 ppm were less than those of the chamber controls after week 57.
A spectrum of non-neoplastic lesions was observed in the respiratory and olfactory epithelium (EPI) of the nose in exposed rats. The lesions included suppurative inflammation, ulceration of the respiratory EPI, hyaline droplet accumulation in the glands of the respiratory EPI, necrosis of the turbinates, squamous metaplasia of the respiratory EPI, hyperplasia of the respiratory EPI, atrophy of the olfactory EPI, hyaline droplet accumulation in the respiratory and olfactory EPI, basal cell hyperplasia of the olfactory EPI, respiratory metaplasia of the olfactory EPI and goblet cell hyperplasia.
The incidence of chronic inflammation of the pleura was significantly increased in 125 ppm females. In addition, the incidences of histiocytic cellular infiltration of the alveolus of the lung were significantly increased in all the exposed groups of females and the incidence of chronic inflammation was significantly increased in 125 ppm females. In 125 ppm males, the incidence of suppurative inflammation of the cornea was significantly increased.
There were significant differences in accumulation of hyaline droplets in the respiratory epithelial glands, olfactory EPI and respiratory EPI (NTP, Citation2011). These effects were graded as minimal to mild. Minimal to mild changes for accumulation of hyaline droplets are not considered degenerative changes (NTP, Citation2014) (http://ntp.niehs.nih.gov/nnl/respiratory/nose/epaccum/index.htm). Hyaline droplet formation is a common occurrence in aged rodents (Ong et al., Citation2015).
Statistical differences in respiratory metaplasia in olfactory EPI, ulcer in the respiratory EPI; hyperplasia of Goblet cells and necrosis of the turbinate occurred at the highest concentration of 125 ppm only and were not considered as the lowest, critical effects (data not shown). shows the incidences of non-neoplastic lesions of the nose in rats with the lowest LOAEL and NOAEL values that were significantly different from controls and had adequate concentration/response relationships. The LOAEL of 31 ppm from the rat study is based on atrophy in the olfactory EPI and hyperplasia of the respiratory EPI, effects statistically different from controls at 31 ppm (). A NOAEL from the rat study was not identified.
Table 3. Incidences of lesions of the nose in rats in 105-week inhalation study.
105-week study in mice
Groups of 50 male and 50 female mice were exposed to DEA vapor at concentrations of 0 (control), 16, 31 or 62.5 ppm, 6 h/day, 5 days/week for 105 weeks (NTP, Citation2011). Survival of exposed groups of mice was similar to that of the chamber control groups. Mean body weights of males and females were similar to those of the chamber controls. Eye abnormality was observed in greater incidence in exposed groups of males than in the chamber controls, and torso/ventral ulcer/abscess was observed in six 62.5 ppm exposed males compared with none in the chamber controls.
A similar spectrum of non-neoplastic lesions was seen in the nose of exposed mice as was seen in rats, although mice were slightly more sensitive. The lowest concentration of mice were exposed (16 ppm) was 2-fold lower than the lowest concentration rats were exposed (31 ppm).
There were significant differences in accumulation of hyaline droplets in the respiratory epithelial glands and respiratory EPI. These effects were graded as minimal to mild. Minimal to mild changes for accumulation of hyaline droplets are not considered degenerative changes (NTP, Citation2014) (http://ntp.niehs.nih.gov/nnl/respiratory/nose/epaccum/index.htm). Hyaline droplet formation is a common occurrence in aged rodents (Ong et al., Citation2015).
Adverse effects that were statistically different from controls at the highest concentration of 62.5 ppm only are as follows: hyperplasia of glands, respiratory EPI; chronic active inflammation of the glands of the respiratory EPI; suppurative inflammation and necrosis of the respiratory EPI (data not shown). These effects were not considered as the lowest, critical effects.
shows the incidences of non-neoplastic lesions of the nose in mice with the lowest LOAEL and NOAEL values that were significantly different from controls and had adequate concentration/response relationships and increases in severity grades. The effects that occurred at the lowest concentration of 16 ppm from the mouse study are as follows: (1) atrophy in the olfactory EPI, (2) respiratory metaplasia in the olfactory EPI and (3) hyperostosis in the turbinates (increase in the amount of bone resulting in a thickened anatomic structure). These effects had the best concentration response in both number of animals affected and increasing severity of lesions (minimal to moderate).
Table 4. Incidences of lesions of the nose in mice in 105-week inhalation study.
14-week study
Groups of 10 male and 10 female rats/mice were exposed to DEA vapor at concentrations of 0 (control), 8, 16, 32, 62 or 125 ppm, 6 h/day, 5 days/week for 14 weeks (NTP, Citation2011). There was no evidence of systemic toxicity associated with DEA exposure for 14 weeks except for mice. The mean body weights of 125 ppm male and female mice were significantly less than those of the chamber controls. There were no exposure-related changes in hematology, serum chemistry indices or organ weights of exposed rats or mice.
NTP (Citation2011) evaluated reproductive tissues and estrous cycle characterization in both rats and mice after the 14-week exposure. At the end of the studies, sperm samples were collected from male animals in the 0, 32, 62 and 125 ppm groups for evaluations. The following parameters were evaluated: spermatid heads per testis and per gram testis, spermatid counts and epididymal spermatozoal motility and concentration. The left cauda, left epididymis and left testis were weighed. Vaginal samples were collected for up to 12 days prior to the end of the investigations from females exposed to 0, 32, 62 or 125 ppm for vaginal cytology evaluations. The percentage of time spent in the various estrous cycle stages and estrous cycle length were evaluated.
14-week rat study
In rats, all the reproductive tissues and estrous cycle characterization parameters were not significantly different from controls. There were no significant differences in the lengths of estrous cycles between chamber control and exposed groups of females. There were significant exposure concentration-related decreases in percent sperm motility in males at 32, 62 and 125 ppm ().
Table 5. Concentration-dependent decrease in percent sperm motility from a 14-week study (NTP, Citation2011).
Exposure-related nasal lesions were seen primarily in rats exposed to 62 or 125 ppm. These lesions included turbinate necrosis, suppurative inflammation, respiratory epithelial hyperplasia, squamous metaplasia of the respiratory EPI and olfactory epithelial atrophy. The LOAEL was 32 ppm for decreases in sperm motility and the LOAEL and NOAEL were 62 and 32 ppm, respectively, for atrophy of the olfactory EPI and hyperplasia of the respiratory EPI.
14-week mice study
In mice, reproductive tissues and estrous cycle characterization parameters were not significantly different from controls, except for the following: (1) there were significant exposure concentration-related decreases in sperm motility in males exposed to 32 , 62 or 125 ppm () and (2) the estrous cycle of 125 ppm females was significantly longer than that of the chamber controls, but only by half a day.
Histopathologic changes in the 62 or 125 ppm groups were noted primarily in the nasal cavity and involved both the respiratory and olfactory EPI of males and females principally. At 125 ppm, lesions included suppurative inflammation, squamous metaplasia of the respiratory EPI, olfactory epithelial atrophy and necrosis of the turbinates. Incidences of olfactory epithelial atrophy in both males and females were significantly increased at 32 ppm and above. The LOAEL of 32 ppm was the same for both decreases in sperm motility and olfactory epithelial atrophy.
Mode-of-action analysis and dose metric
Diethylamine is corrosive and strongly alkaline, with a pKa of 11.09 (HSDB, Citation2015). When amines with a high pKa come in contact with tissues or fluids at physiologic pH, they become protonated and hydroxide ion is released, causing local necrosis (). DEA is irritating to the skin and mucous membranes (NTP, Citation2011). The mechanism for hyperostosis observed in mice was discussed by NTP (Citation2011). The proposed mechanism is based on DEA’s strongly alkaline properties resulting in an imbalance of normal bone remodeling activity. This may be associated with decreased bone resorption from neutralization of the normally acidic osteoclast resorption pit. The MOA for decrease in sperm motility is unknown. The exposure concentration of DEA was used as the dose metric since point-of-entry effects were observed at the lowest concentrations. DEA is assumed to have a threshold MOA and the critical effects are relevant to humans.
Identification of the POD
The TCEQ performed Benchmark Concentration (BMC) modeling using USEPA Benchmark Dose software (version 2.4, obtained from USEPA, Washington, DC) for the data in and , which were taken from the NTP (Citation2011) rat and mouse study. Data were used to predict the central estimate (Benchmark Concentration, BMC10) and the 95% lower confidence limit on the BMC (BMCL10) using dichotomous models. A default benchmark response (BMR) of 10% was selected for extra risk for the BMC10 and BMCL10. BMC modeling was conducted on the combined male and female data for each of the histological endpoints that showed a dose–response relationship and had at least two recorded incidences past the control value, making them suitable for BMC modeling. All the available dichotomous models were run. The best-fit models for each of the histological end points are listed in along with their detailed results. Data for atrophy of rat olfactory EPI were not amenable to BMC modeling (the dose–response was too steep), so the LOAEL of 31 ppm was used as the POD ().
Table 6. BMC results for the best-fit model for the examined endpoints.
Decrease in sperm motility data from was modeled using continuous models for both rats and mice. A default BMR of 1 SD was used (the standard BMR for continuous data; TCEQ, Citation2015b). However, data for decreases in sperm motility were not amenable to continuous BMC modeling. Therefore, the POD for decrease in sperm motility is the LOAEL of 32 ppm ().
provides a summary of results for BMC modeling for adverse effects. TCEQ (Citation2015a) provides additional details on BMC modeling including figures of the BMC curves. Generally, the rat had higher LOAELs, BMCs and BMCL10 compared with the mouse, except for hyperplasia of the respiratory EPI in rats with a BMC of 4.23 ppm and BMCL of 3.66 ppm. However, the average severity of hyperplasia of respiratory EPI in rats at 31 ppm, the LOAEL value, were lower than or equal to the control value and were of minimal severity (). Therefore, hyperplasia of respiratory EPI in rats was not considered as the critical effect. Hyperostosis in the turbinates in mouse had the best concentration response in both number of animals affected and increasing severity of lesions (mild to moderate) with the lowest LOAEL of 16 ppm (). Therefore, hyperostosis in the turbinates in mice was chosen as the critical effect. NTP (Citation2011) noted that the mouse was more sensitive than the rat.
The BMC10 of 9.13 ppm and BMCL10 of 5.71 ppm for hyperostosis in the turbinates observed in mice are lower than the LOAEL of 32 ppm for decrease in sperm motility (). The LOAEL for decrease in sperm motility occurred at the same concentration as upper respiratory effects (atrophy of the olfactory EPI). Therefore, if effects in the respiratory tract are prevented, then reproductive effects in male mice and rats will be prevented.
Critical effect and POD
NTP (Citation2011) regarded the mouse as the most sensitive species and the TCEQ agrees with this observation. Therefore, the critical effect is hyperostosis in the turbinates observed in the mouse with a BMCL10 value of 5.71 ppm. Since duration adjustments and dosimetric adjustments are identical for the rat and mouse POD, identifying the critical effect of hyperostosis in the turbinates observed in mice is defensible at this stage of the assessment.
Duration adjustments
The effects of DEA are assumed to be concentration- and duration-dependent. An adjustment from a discontinuous to continuous exposure duration was conducted (TCEQ, Citation2015b) as follows:
(1)
where D = exposure duration (h per day) and F = exposure frequency (days per week). The PODADJ = 5.71 ppm × (6/24) × (5/7) = 1.020 ppm.
Dosimetric adjustments from animal-to-human exposure
The health effects produced by DEA at lower concentrations are respiratory tract effects in the extrathoracic region of the respiratory tract, so default dosimetric adjustments were performed as a Category 1 vapor based on updated recommendations in USEPA (Citation2012) in order to calculate a human equivalent PODHEC. For Category 1 gases, the default dosimetric adjustment from animal-to-human exposure is conducted using the following equation:
(2)
A default value of 1 was used for the Regional Gas Dose Ratio (RGDR) for a Category 1 gas with extrathoracic respiratory effects (USEPA, Citation2012).The PODHEC is 1.020 ppm × 1 = 1.020 ppm or 1020 ppb.
Application of uncertainty factors
The lowest PODHEC of 1020 ppb from the NTP (Citation2011) chronic study was based on hyperostosis in the turbinates in the mice. The default for non-cancer effects is to determine a PODHEC and apply UFs to extrapolate from the POD to lower concentrations (i.e. assume a threshold MOA) in order to calculate a ReV.
To calculate the chronic ReV, the following UFs were applied: intrahuman (UFH), animal-to-human (UFA), LOAEL-to-NOAEL (UFL) and database completeness (UFD). A UFH of 10 was used to account for variation in sensitivity among members of the human population. The TCEQ believes that a UFH of 10 is sufficient to account for human variation including possible child/adult differences. A UFA of 3 was used because default dosimetric adjustments from animal-to-human exposure was conducted, which accounts for toxicokinetic differences but not toxicodynamic difference. A UFL of 1 was used because BMC modeling was conducted and the resulting POD (BMCL10) is considered a NOAEL.
A UFD of 3 was used because a one-generation or two-generation reproductive study was not available. The 14-week reproductive study conducted in rats and mice (NTP, Citation2011) indicated possible effects on male fertility (i.e. a decrease in sperm motility). However, in mice, effects on the upper respiratory tract occurred at the same concentration as a decrease in sperm motility. Short-term developmental toxicity data in experimental animals or humans were not found in the literature. In general, the amine chemical class has not been shown to cause developmental effects (OECD, Citation2011, Citation2013). A higher UFD was not used because MOA information was available and deemed to be relevant for humans. There is one well-conducted chronic study conducted in both rats and mice (NTP, Citation2011) and a chronic study in rats (Lynch et al., Citation1986). The total UFs applied to the PODHEC is 90.
(3)
Health-based chronic ReV
The chronic ReV was rounded to two significant figures. The resulting chronic ReV is 11 ppb (33 µg/m3). summarizes the different steps of the toxicity assessment. provides a summary of the confidence in the different steps of the chronic toxicity assessment based on guidance in Grant et al. (Citation2015). Database completeness was medium and study quality was high. The overall confidence in the toxicity assessment was medium to high.
Table 7. Derivation of the chronic ReV.
Table 8. Confidence in the chronic toxicity assessment.
Discussion
The TCEQ (Citation2015a) conducted a chronic non-cancer toxicity assessment for DEA, a secondary amine, in order to develop a chronic inhalation ReV for DEA. The chronic ReV of 11 ppb (33 µg/m3) is based on a well-conducted toxicity study in rodents (NTP, Citation2011). The confidence in the toxicity assessment was medium to high. The critical effect was hyperostosis in the turbinates in mice although numerous other non-neoplastic lesions were observed. In the 14-week study, a decrease in sperm motility occurred at the same concentration as atrophy of the EPI in mice. A decrease in sperm motility was the only systemic effect observed at lower concentrations. A decrease in sperm motility has been highly correlated with an adverse effect on male fertility (Morrissey et al., Citation1988). However, if respiratory effects are prevented then male reproductive effects may not occur. There are no developmental toxicity investigations for DEA, but the amines as a chemical group do not produce developmental effects (OECD, Citation2011, Citation2013). Respiratory effects observed in rodents are relevant to humans based on the MOA.
The TCEQ also developed an acute ReV for DEA for evaluation of 1-h data (TCEQ, Citation2015a). The acute ReV was based on a human clinical study reported by Lundqvist et al. (Citation1992). The critical effect was low to moderate nasal and eye irritation in five non-smoking males exposed to an average DEA concentration of 10 ppm (0–12 ppm for 60 min). UFs totaling 90 were applied to the human POD to calculate the DEA acute ReV of 110 ppb (330 µg/m3). Whereas the acute ReV for DEA is based on sensory irritation effects in humans, the chronic ReV is based on cellular damage in the nasal region in rodents.
NTP (Citation2011) reviewed the evidence that DEA causes cancer. Although data from mammalian cell studies were limited, NTP found no evidence of genotoxicity by DEA in the scientific literature. Epidemiology studies or case reports in humans examining DEA exposure and cancer risk were not located in the literature. Limited oral studies in animals indicated no carcinogenic potential (NTP, Citation2011). Under the conditions of NTP’s 2-year inhalation study, there was no evidence of cancer activity in rodents.
The Texas Clean Air Act mandates the State of Texas to prevent air pollution from causing adverse health and welfare effects (TCEQ, Citation2015b). The TCEQ relies on its extensive air permitting and air monitoring programs for permitting, surveillance and compliance initiatives to verify that concentrations of chemicals in ambient air do not exceed safe levels (Capobianco et al., Citation2013). DEA is not monitored for by the TCEQ’s ambient air monitoring program. However, Key et al. (Citation2011) measured concentrations of DEA from non-detect to 28 part per trillion by volume in ambient air from Hermon Park (Los Angeles, CA). These measured ambient air levels are well below DEA’s acute and chronic ReVs of 110 and 11 ppb, respectively.
TCEQ conducts chemical-specific toxicity assessments for all permitted chemicals to assess safe levels in air. A similar analytical approach used for DEA was used for hazard identification and dose–response assessment to derive acute and chronic ReVs for 1,3-butadiene (Grant et al., Citation2010), ethylene (Erranguntla & Grant, Citation2015), hexamethylenediamine (Myers & Grant, Citation2015) as well as numerous other chemicals (Final Development Support Documents at http://www.tceq.state.tx.us/toxicology/dsd/final.html).
For air permitting, the acute and chronic ReVs are reduced by 70% to calculate the short-term DEA Effects Screening Level (ESL). The ESL used in air permitting is lower than the ReV to account for cumulative and aggregate exposure (Capobianco et al., Citation2013; TCEQ, Citation2015b). The short-term ESL is 33 ppb (99 µg/m3) and long-term ESL is 3.3 ppb (9.9 µg/m3) for DEA. Both the short- and long-term ESL for DEA are adequately health-protective, and will be used during the review of air permit applications during health effects evaluation of modeled short-term and annual-averaged ambient air data. The ESLs will be used in the TCEQ air permitting process to assess the protectiveness of substance-specific emission rate limits for facilities undergoing air permit reviews. If the acute and chronic maximum-ground-level concentration, a worst case-modeled concentration resulting from a worst case emission rate, is below the short-term or long-term ESL, respectively, for that chemical, then the substance can be judged, with reasonable confidence, to present a low probability of risk.
A Screening Information Data Set (SIDs) for aliphatic secondary amines was recently proposed by OECD (Citation2013). OECD (Citation2013) states “The Aliphatic Secondary Amines category is represented by the structure R–NH–R’, where R is an alkyl group that may be linear or branched. The alkyl group may include an atom or group that will not react with or substantially affect the properties of the amine function. The tendency to share the non-bounded electron pair on the nitrogen underlies the chemical behavior of amines as a group”. There are nine sponsored aliphatic secondary amines in this group:
dimethylamine (CAS# 124-40-3)
DEA (CAS# 109-89-7)
dipropylamine (CAS# 142-84-9)
diisopropylamine (CAS# 108-18-9)
dibutylamine (CAS# 111-92-2)
diisobutylamine (CAS# 110-96-3) and
three isomers of dipentylamine (CAS# 2050-92-2, 61361-18-0 and 27094-65-1).
Only a few of these secondary amines have chronic toxicity data. The TCEQ plans to investigate whether the DEA toxicity assessment may be used to develop chronic toxicity values for other secondary amines in this group based on a read-across or structural analog approach (TCEQ, Citation2015b). The first step in this process was to develop chronic toxicity values for DEA, a chemical with adequate chronic toxicity data.
Acknowledgements
We would like to acknowledge Dr Tiffany Bredfeldt for the preparation of and Dr Jessica Myers for conducting BMC modeling.
Declaration of interest
The authors report no declarations of interest.
References
- American Conference of Governmental Industrial Hygienists (ACGIH). (2001). Diethylamine. Documentation of the threshold limit values and biological exposure indices. 7th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists
- Brieger H, Hodes WA. (1951). Toxic effects of exposure to vapors of aliphatic amines. AMA Arch Ind Hyg Occup Med 3:287–91. (As cited in NTP 2011)
- Capobianco T, Hildebrand SM, Honeycutt M, et al. (2013). Impact of three interactive Texas state regulatory programs to decrease ambient air toxic levels. J Air Waste Manag Assoc 63:507–20
- Erranguntla NE, Grant RL. (2015). Health- and vegetative-based effects screening values for ethylene. Chem Biol Interact (in press). doi: 10.1016/j.cbi.2015.02.010
- Grant RL, Haney J, Curry AL, Honeycutt M. (2010). A chronic reference value for 1,3-butadiene based on an updated noncancer toxicity assessment. J Toxicol Environ Health B Crit Rev 13:460–75
- Grant RL, Santos SL, Dourson ML, et al. (2015). Unpacking toxicity assessments to understand and improve confidence. In: The Toxicologist: Supplement to Toxicological Sciences, 144, Society of Toxicology, 2015, Abstract no. 873. [Presentation available at http://blog.americanchemistry.com/wp-content/uploads/2015/04/Grant-SOT-2015-Final.pdf. [Last accessed: 14 Jul 2015]
- Grant WM. (1986). Toxicology of the eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 76
- Hazardous Substance Data Base (HSDB). (2015). Diethylamine. [Last accessed: 15 Jun 2015]
- Key D, Stihle J, Petit J-E, et al. (2011). Integrated method for the measurement of trace nitrogenous atmospheric bases. Atmos Meas Tech 4:2795–807
- Lundqvist GR, Yamagiwa M, Pedersen OF, Nielsen GD. (1992). Inhalation of diethylamine-acute nasal effects and subjective response. Am Ind Hyg Assoc J 53:181–5
- Lynch DW, Moorman WJ, Stober P, et al. (1986). Subchronic inhalation of diethylamine vapor in Fischer-344 rats: organ-system toxicity. Fundam Appl Toxicol 6:559–65
- Mackison FW, Stricoff RS, Partridge LJ Jr. (eds). (1981). NIOSH/OSHA – occupational health guidelines for chemical hazards. DHHS (NIOSH) Publication No. 81-123 (3 VOLS). Washington, DC: U.S. Government Printing Office, 1
- Montelius J (ed.). (2013). Scientific basis for Swedish Occupational Standards XXXII. Swedish criteria group for occupational standards. Arbete Och Hälsa 47:1–15
- Morrissey RE, Lamb JC IV, Schwetz BA, et al. (1988). Association of sperm, vaginal cytology, and reproductive organ weight data with results of continuous breeding reproduction studies in Swiss (CD-1) mice. Fundam Appl Toxicol 11:359–71
- Myers J, Grant RL. (2015). Development of a chronic inhalation reference value for hexamethylenediamine using an exposure model based on the dihydrochloride salt. Inhal Toxicol 27:440–9
- National Institute for Occupational Safety and Health (NIOSH). (1987). Occupational cardiac toxicity – acute diethylamine exposures. NIOSH Study No. CAN 339. Prepared by Experimental Pathology Laboratories, Inc., for the National Institute for Occupational Safety and Health, Cincinnati, OH. (As cited in NTP 2011)
- National Institute of Occupational Safety and Health (NIOSH). (2011). NIOSH pocket guide to chemical hazards. Available from: http://www.cdc.gov/niosh/npg/npgd0209.html. [Last accessed: 2 Jul 2014]
- National Research Council (NRC). (1983). Risk assessment in the federal government. Washington, DC: National Research Council, National Academy Press
- National Research Council (NRC). (1994). Science and judgment in risk assessment. Washington, DC: National Research Council, National Academy Press
- National Research Council (NRC). (2001). Standing operating procedures for developing acute exposure guideline levels for hazardous chemicals. Washington, DC: National Research Council, National Academy Press
- National Toxicology Program (NTP). (2011). NTP technical report on the toxicology and carcinogenesis studies of diethylamine (Cas no. 109-89-7) in F344/n rats and B6C3F1 mice (inhalation studies). Research Triangle Park, NC: U.S. Department of Health and Human Services, National Institutes of Health. NTP TR 566, NIH Publication No. 12-908 http://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr566.pdf [accessed June 24, 2014]
- National Toxicology Program (NTP). (2014). Nonneoplastic lesion atlas. A guide for standardizing terminology in toxicologic pathology for rodents. In: Cesta MF, Malarkey DE, Herbert R, Brix A, Sills RC. (eds.) Research Triangle Park, NC: U.S. Department of Health and Human Services, National Institutes of Health. Available from: http://ntp.niehs.nih.gov/nnl/index.htm. [Last accessed: 10 Aug 2015]
- Neurath GB, Dünger M, Pein FG, et al. (1977). Primary and secondary amines in the human environment. Food Cosmet Toxicol 15:275–82
- Ong CB, Kumagai K, Brooks PT, et al. (2015). Ozone-induced type 2 immunity in nasal airways: development and lymphoid cell dependence in mice. Am J Respir Cell Mol Biol (in press)
- Organisation for Economic Co-operation and Development (OECD). (2011). 201 SIAM 32, 19–21 April 2011. US/ICCA. SIDS initial assessment profile, chemical category C1-13 primary amines. Available from: http://webnet.oecd.org/Hpv/UI/handler.axd?id=9e86965a-715b-4cb8-99a4-f7113a364ea9. [Last accessed 14 Apr 2015]
- Organisation for Economic Co-operation and Development (OECD). (2013). CoCAM4, 16-18 April 2013. US/ICCA. SIDS initial assessment profile, aliphatic secondary amines. Available from: http://webnet.oecd.org/hpv/ui/handler.axd?id=D79CCEC2-4FF0-4DA5-88DE-9690EA9F3FF9. [Last accessed 28 April 2015]
- Texas Commission on Environmental Quality (TCEQ). (2015a). Development support document for diethylamine, CAS Registry Number: 109-89-7. Austin, TX: Office of the Executive Director
- Texas Commission on Environmental Quality (TCEQ). (2015b). TCEQ guidelines to develop toxicity factors (Revised RG-442). Austin, TX: Office of the Executive Director
- Uhegbu FO, Maduagwu EN. (1995). Occurrence of nitrosatable amines in some Nigerian medicinal plants. Bull Environ Contam Toxicol 55:643–9
- Union Carbide. (1950). Range finding tests on diethylamine. U.S. EPA/OTS Public Files. Document No. 86-870001410. Fiche No. 0515572. (As cited in NTP 2011)
- U.S. Environmental Protection Agency (USEPA). (1994). Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. EPA/600/8–90/066F. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development
- U.S. Environmental Protection Agency (USEPA). (2002). A review of the reference dose and reference concentration processes. EPA/630/P-02/002F. Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum
- U.S. Environmental Protection Agency (USEPA). (2012). Advances in inhalation gas dosimetry for derivation of a reference concentration (RfC) and use in risk assessment. EPA/600/R-12/044. Washington, DC: United States Environmental Protection Agency
- Virginia Chemicals. (1987). Pathologic findings in Fischer 344 rats exposed by inhalation to allylamine, ethylamine, diethylamine, and triethylamine with cover letter dated 042484. OTS # 308080. Doc # 86-870000813. Fiche # 0515251. (Animal data sheets from Dr. Dennis Lynch attached.)
