Abstract
Scleritis is an ocular inflammatory disorder commonly associated with systemic autoimmune diseases. We report a case of nodular scleritis with an etiological diagnosis of tuberculosis wherein diagnosis was possible only after histopathological examination of the enucleated eye. Method of study: A 52 year female patient was referred as a case of nodular scleritis not responding to topical and oral anti-inflammatory agents. She was being treated with immunosuppressives for rheumatoid arthritis by her rheumatologist. Scleritis improved initially but worsened in few months with development of complications. Eye was enucleated and histopathological examination revealed tuberculous bacilli in retinal pigment epithelial cells. Conclusion: Infective scleritis should be suspected in cases of scleritis which progress despite treatment. Reactivation of latent Mycobacterium tuberculosis may occur especially in patients on long term systemic immunosuppressive treatment. Early detection and aggressive treatment is necessary for preventing morbidity or mortality due to these infections.
We report a case of tuberculous scleritis in which diagnosis was made only after histopathological examination and applying polymerase chain reaction (PCR) on tissue specimens. A 52-year-old woman from south India was referred to us with a history of pain in her left eye associated with redness of 3 months duration and with a diagnosis of nodular scleritis not responding to topical medications. She was diagnosed to have rheumatoid arthritis and was on treatment for the past 3 months with oral prednisolone 10 mg/day, and oral methotrexate 25 mg per week by her rheumatologist. Her best-corrected distance visual acuity was 6/6 and near vision was N6 in both eyes. Examination revealed marked scleral injection with a scleral nodule superiorly in the left eye. The rest of the ocular examination was unremarkable. Investigations done by her rheumatologist showed rheumatoid factor was positive and anti-nuclear antibodies and antineutrophilic cytoplasmic antibodies were negative. Total WBC count was 16,000 cells/mm3. Erythrocyte sedimentation rate was elevated and chest radiograph was normal.
We started oral indomethacin sustained release preparation 75 mg twice daily and topical prednisolone eyedrops in tapering doses along with her systemic immunosuppressive medications. She showed initial signs of improvement but 3 months later scleral necrosis and staphyloma with anterior chamber cells and flare were noted. Intravenous methyl prednisolone 1 g per day was given for 3 days. Tablet cyclophosphamide 100 mg per day was started and oral prednisolone 10 mg per day was continued.
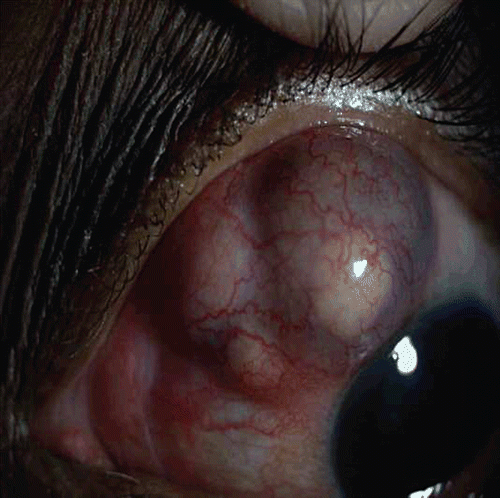
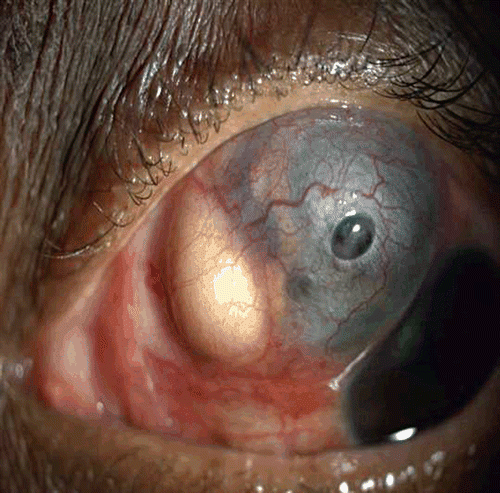
She was lost to follow-up and after 3 months presented with severe pain and decreasing vision, having stopped all medications. Examination of the left eye showed necrotizing scleritis with pus points, exudates in the anterior chamber (). Her vision had reduced to no light perception. Since it was painful, the left eye was enucleated.
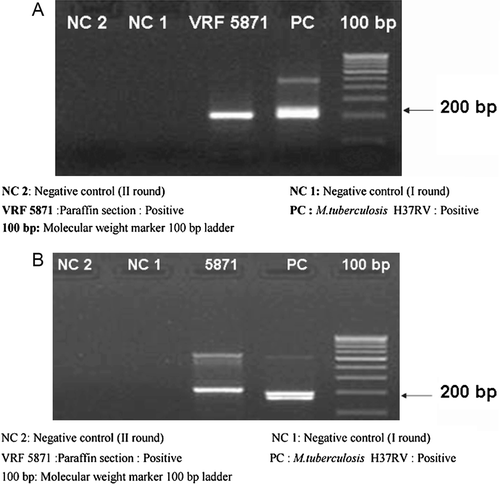
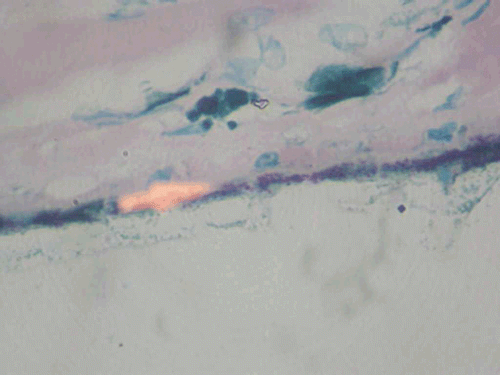
Histopathological examination of the enucleated eye showed corneal edema, exudative membrane over the iris, dense inflammatory exudates in the vitreous, and choroid with granuloma comprising giant cells. Scleral necrosis and suppurative inflammation were seen. Acid-fast bacilli were seen in the retinal pigment epithelial cells and a diagnosis of necrotizing granulomatous inflammation due to tuberculosis was made (, ). Polymerase chain reaction of the specimen targeting the IS6110 region and MPB64 gene revealed Mycobacterium tuberculosis genome (). The patient was referred to an infectious disease specialist for systemic evaluation and management. Subsequently, she developed symptoms and signs of meningitis and was found to have tuberculous meningitis, which was diagnosed and treated by her neurologist.
FIGURE 3 Histopathological examination of the specimen showing multiple granulomas in the sclera. Hematoxylin and eosin, ×100.

DISCUSSION
Scleritis is the inflammatory disease of the sclera most commonly associated with systemic autoimmune diseases. Rheumatoid arthritis is the most commonly associated disease, followed in incidence by Wegener granulomatosis, relapsing polychondritis, inflammatory bowel disease, and systemic lupus erythematosus. Anterior scleritis caused by infections was reported in about 4–18% of cases in a study.Citation1
Infectious scleritis can be viral, bacterial, fungal, and parasitic and commonly occurs following trauma or surgeries. Pterygium surgery with adjuvant mitomycin C or beta irradiation, retinal surgeries, especially scleral buckling for retinal detachment, cataract extraction surgery, and surgical procedures for strabismus are reported to cause infectious scleritis.Citation2–5
Untreated, primary or secondary infectious scleritis may result in loss of the eye through extension of the infection to adjacent structures and eventual phthisis or perforation. Infection with Pseudomonas species, Staphylococcus aureus, or herpes zoster can cause necrotizing scleritis that may be clinically similar to that caused by systemic autoimmune disease. In a study of scleritis patients 11 out of 55 cases were due to infections.Citation6 The most common cause of infectious scleritis is Pseudomonas aeruginosa. Other organisms include Streptococcus spp., Staphylococcus spp., Aspergillus spp., Acanthamoeba spp., Proteus mirabilis, and herpes zoster. Infections such as toxoplasmosis, syphilis, tuberculosis, and toxocariasis have also been reported to cause scleritis.Citation7–12 A case of necrotizing nocardial scleritis with intraocular extension is reported.Citation13 Reynolds and Alfonso reported infectious scleritis in a patient with acquired immunodeficiency syndrome.Citation14
Infectious scleritis after systemic immunosuppressive therapy is reported.Citation15 The diagnosis of infective scleritis may be mistaken for a necrotizing scleritis secondary to rheumatoid arthritis or other collagen vascular diseases and treatment may be delayed. Patients on systemic immunosuppressive agents have bone marrow suppression and severe infections are found to develop in these patients. Reactivation of latent tuberculosis is also reported in these patients.
A case has been reported of atypical infectious nodular scleritis with severe necrotizing inflammation and scleral rupture where diagnosis was confirmed by real-time polymerase chain reaction, which revealed tuberculosis genome.Citation16 Mycobacterium tuberculosis has been identified in the retinal pigment epithelium in a case of tuberculous uveitis.Citation17 Identification of latent tuberculosis is important prior to starting the patients on systemic immunosuppressive treatment. The initial evaluation should include a history to ascertain any risk factors for tuberculosis, and a workup for tuberculosis in the form of chest radiograph and a tuberculin skin test.
CONCLUSIONS
Scleritis is commonly associated with autoimmune diseases; nevertheless infections need to be considered. This case is reported for the fact that scleritis in a patient with rheumatoid arthritis and on systemic immunosuppression with initial presentation as nodular scleritis later worsened despite treatment. The diagnosis of tuberculous scleritis could be made only after histopathological examination of the enucleated eye. Failure to consider tuberculosis in the differential diagnosis of intraocular inflammation may have catastrophic consequences, as the immunosuppressive agents often used to manage intraocular inflammation may be sight or life threatening in patients with active systemic tuberculosis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Schwam BL, Raizman MD, Krachmer JH, Mannis MJ, Holland EJ. Cornea. St. Louis, MO: Mosby; 1997:1479–1491.
- Sainz de la Maza M, Hemady RK, Foster CS. Infectious scleritis: report of four cases. Doc Ophthalmol. 1993;83:33–41.
- Sainz de la Maza M, Foster CS. Necrotizing scleritis after ocular surgery. Ophthalmology. 1991; 98: 1720.
- Hsiao CH, Chen JJ, Huang SC, Ma HK, Chen PY, Tsai RJ. Intrascleral dissemination of infectious scleritis following pterygium excision. Br J Ophthalmol. 1998;82:29–34.
- Zinn KM, Ferry AP. Massive scleral necrosis from a Pseudomonas infection following scleral buckling and pars plana vitrectomy surgery. Mt Sinai J Med. 1980; 47: 618.
- Riono WP, Hidayat, AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases.
- Altman AJ, Cohen EJ, Berger ST, Mondino BJ. Scleritis and Streptococcus pneumoniae. Cornea. 1991;10:341–345.
- Stenson S, Brookner A, Rosenthal S. Bilateral endogenous necrotizing scleritis due to Aspergillus oryzae. Ann Ophthalmol. 1982;14:67–72.
- Lindquist TD, Fritsche TR, Grutzmacher RD. Scleral ectasia secondary to Acanthamoeba keratitis. Cornea. 1990;9:74–76.
- Hemady R, Sainz de la Maza M, Raizman MB, Foster CS. Six cases of scleritis associated with systemic infection. Am J Ophthalmol. 1992;114:55–62.
- Schuman JS, Weinberg RS, Ferry AP, Guerry RK. Toxoplasmic scleritis. Ophthalmology. 1988;95:1399–1403.
- Rodriguez-Ares MT, De Rojas Silva MV, Pereiro M, et al. Aspergillus fumigatus scleritis. Acta Ophthalmol Scand. 1995; 73:467–469.
- Choudhry S, Rao S,K Biswas, J, Madhavan HN. Necrotizing nocardial scleritis with intraocular extension: a case report. Cornea. 2000; 19(2): 246–248.
- Reynolds MG, Alfonso E. Infectious scleritis and keratoscleritis: management and outcome. Am J Ophthalmol. 1991; 112: 543.
- Hwang YS, Chen YF, Lai CC, Chen HSL, Hsiao CH. Infectious scleritis after use of immunomodulators Arch Ophthalmol. 2002;120:1093–1094.
- Kesen MR, Edward DP, Rao NA, et al. Atypical infectious nodular scleritis. Arch Ophthalmol. 2009;127(8):1079–1080.
- Saraswathy S, Rao NA, Smith RE. Tuberculous Uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthamol. 2006; 124:1777–1779.