Abstract
Purpose: To present the first reported case of bilateral H1N1-associated acute retinitis and its successful treatment.
Design: Interventional case report.
Methods: A 41-year-old HIV-positive male presented with acute vision loss, panuveitis, and retinitis. A diagnostic and therapeutic vitrectomy with intravitreal injection of vancomycin and ganciclovir and endolaser was performed. One month later, the patient returned with similar symptoms in the fellow eye and underwent the same procedure.
Results: ELISA immunoassay revealed H1N1 antibodies in both the vitreous and serum. PCR for herpes viruses included HSV, CMV, and VZV. Bacterial and fungal cultures were negative. On 1-year follow-up, the vision remained 20/20 in both eyes without evidence of recurrent inflammation.
Conclusions: H1N1 should be included in the differential diagnosis of any patient with a history of recent influenza A (H1N1) infection and acute retinitis. H1N1 may carry a better prognosis than other viruses causing acute retinitis.
Viral retinitis is one of the most common causes of infection involving the posterior segment and is a potentially visually devastating disease affecting both immunocompetent and immunocompromised individuals.Citation1 Viral retinitis may be congenital or acquired, most commonly due to cytomegalovirus.Citation2 Ocular involvement in cytomegalovirus has been reported to be between 12 and 46% of patients with acquired immune deficiency syndrome (AIDS).Citation3 Other documented causes of ophthalmic manifestations of systemic viral disease include herpes zoster, herpes simplex, and Epstein-Barr virus.Citation4 Influenza A has also been implicated in few reports of ocular findings in systemically infected individuals, with potential findings of influenza A retinopathy, macular edema, foveal irregularity,Citation5 and optic neuritis.Citation6
H1N1 (avian influenza A, swine flu) hit the United States in April 2009, when a 10-year-old patient in California was diagnosed with a influenza strain variant.Citation7 Patients infected with H1N1 may present with flu symptoms such as fever, cough, and body aches. Eye involvement has rarely been reported—limited to one case of presumed H1N1 retinopathyCitation8 and a report of a child who developed bilateral acute anterior uveitis, papillitis, and neuroretinitis after a respiratory infection of H1N1.Citation9 Treatment of influenza caused by H1N1 has proven difficult as resistance is quite high. According to the Centers of Disease Control (CDC), in 2007, resistance to the commonly used anti-virals amantadine and rimantadine was nearly 100%. In 2009, resistance to oseltamavir was noted to also be near 100%.Citation10
The aim of this case report is to present a patient with bilateral acute retinitis, likely secondary to H1N1, who was successfully treated with vitrectomy and intravitreal injection of vancomycin and ganciclovir.
CASE REPORT
A 41-year-old HIV-positive male suffered from redness, pain, and progressive decreased vision in his right eye for several days. Medical history was significant for a CD4 count of 560, undetectable viral load, and recent H1N1 influenza, documented and confirmed by his primary care physician with a rapid influenza diagnostic test, and treated with systemic oseltamivir approximately 3 weeks prior to presentation to our service. Patient's visual acuity was 20/125 in the right eye and 20/20 in the left eye. The right eye demonstrated considerable conjunctival injection and chemosis. The anterior chamber revealed dense inflammation, hypopyon, fibrin, and posterior synechiae. Funduscopic examination revealed a dense vitritis and necrosis of the peripheral retina in the right eye (). The left eye was unremarkable.
Diagnostic as well as therapeutic pars plana vitrectomy surgery was performed on the right eye with an intravitreal injection of 1 mg/0.1 mL vancomycin, and 2 mg/0.1 mL ganciclovir. Prophylactic endolaser was also performed surrounding the retinitis, as demonstrated in the intraoperative video (). A vitreous biopsy revealed negative herpes viruses (HSV, VZV, and CMV) PCR acid-fast bacilli, and bacterial and fungal cultures. The patient was also prescribed 1 g of oral valacyclovir 3 times per day by the vitreoretinal surgeon.
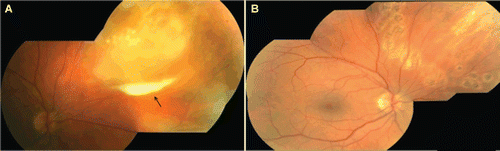
FIGURE 1 (A) Mosaic color fundus photograph of the right eye, demonstrating focal and confluent areas of necrosis of the peripheral retina. Preretinal exudate, a “pseudohypopyon,” is also shown in addition to areas of retinal necrosis (arrow). (B) Mosaic color fundus photograph of the right eye after pars plana vitrectomy and laser retinopexy treatment, showing resolved retinal necrosis.
One week postoperatively, the vision in the right eye improved to 20/30 and pain had resolved. However, within 2 weeks the patient returned for a follow-up visit complaining of swelling, pain, and floaters in his fellow eye. Vision was 20/20 in both eyes. Examination of the anterior chamber revealed mild conjunctival infection and moderate inflammation in the left eye. Funduscopic examination demonstrated focal coalescent necrosis of the retina and vitreous haze, indicating retinitis in the fellow eye. The right eye remained stable.
The patient underwent a 25-gauge pars plana vitrectomy surgery in the left eye and intravitreal injection of the same doses of antibiotics and antiviral agents. One week postoperatively, the patient's vision was 20/20 in both eyes and retinal necrosis was completely resolved (). Vitreous herpes viruses (HSV, VZV, and CMV) PCR acid-fast bacilli, and bacterial and fungal cultures were negative. However, serum and vitreous ELISA immunoassay for influenza A (H1N1) antibodies were positive. At a 1-year follow-up examination, vision remained 20/20 in both eyes without evidence of recurrent intraocular inflammation. The patient is currently continuing his HIV medications only, as prescribed by his internist.
Although another herpetic viral cause of our patient is certainly high on the differential in this HIV-positive individual, our patient's rapid response to intravitreal anti-viral therapy, despite a negative viral workup, alludes to a viral cause, specifically H1N1, of his acute retinitis. The patient was on systemic valacyclovir when he developed contralateral retinitis, suggesting H1N1 rather than another virus as a cause for the retinitis. It is our belief that our patient, who suffered from viral retinitis, traditionally a devastating and blinding disease, had a favorable outcome due to several factors. Firstly, the patient sought treatment immediately upon experiencing visual symptoms. Secondly, his general ophthalmologist had a high index of suspicion for an infectious endophthalmitis and referred him immediately to a retina specialist. The patient was taken to surgery for a vitreous biopsy and intravitreal injection of broad-spectrum antibacterial and antiviral agents on the same day of consultation, without delay. Because of the acute nature of the retinitis and our suspicion of possible acute retinal necrosis (ARN), early surgery was recommended, yielding an excellent clinical result, despite the grim natural history of ARN. We cannot determine of this favorable prognosis was due to our early surgical intervention or because of the atypical viral cause. However, as small-gauge vitrectomy is available, the threshold for surgical intervention may be lower nowadays than in previous years.
It is plausible that this may be a case of retinitis secondary to a herpesvirus infection, undetected by PCR, although, the sensitivity and specificity of immunodetection of herpesvirus antigens are known to be over 90%.Citation11 The swine influenza virus (SIV) ELISA immunoassay (IDEXX Laboratories, Westbrook, Maine) that was used for the detection of antibodies to swine influenza virus in the serum and in the vitreous has limitations: although highly specific for the detection of H1N1 in serum, cross-reactivity has occurred in porcine serum and plasma to other SIV subtypes. The Goldmann Witmer coefficient was also calculated as (anti-SIV IgG in vitreous/total IgG in vitreous)/(anti-SIV IgG in serum/total IgG in serum) and found to be 2.8. A value of 2 was considered evidence of intraocular antibody synthesis.Citation12 However, sensitivities and specificities of the test to detect H1N1 antibodies in vitreous are currently unknown. Recently, reactivation of acute retinal necrosis from varicella zoster infection after H1N1 vaccination was reported.Citation13 To our knowledge this is the first case to describe H1N1-associated acute retinitis, which may be caused by the H1N1 virus itself or be indicative of a secondary viral cause of acute retinitis undetected by PCR. Because antibodies and not antigens were identified, cross-reactivity to other viral antigens may be a confounding factor.
In summary, we highly recommend that any patient who has a history of recent influenza A (H1N1) infection and visual symptoms be referred immediately to an ophthalmologist. H1N1 may carry a better prognosis than other viruses causing acute retinitis. Without more reported cases, the favorable outcome of H1N1 retinitis as observed by our patient, may, in fact, be due to the natural history of progression of H1N1 retinitis and not a specific treatment.
Supplementary Material
Download (36.6 MB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Biswas J, Madhavan HN, Gopal L, Badrinath SS. Clinical features and virologic studies in viral retinitis. Indian J Ophthalmol. 1995;43(2):63–68.
- Carlstrom G, Alden J, Belfrage S, et al. Acquired cytomegalovirus infection. Br Med J. 1968;2:521–525.
- Freeman WR, Lerner CW, Mines JA, et al. A prospective study of the ophthalmologic findings in the acquired immune deficiency syndrome. Am J Ophthalmol. 1984; 97:133–142.
- Yoser SL, Forster DJ, Rao NA. Systemic viral infections and their retinal and choroidal manifestations. Surv Ophthalmol. 1993:37(5):313–352.
- Mathur SP. Macular lesion after influenza. Br J Ophthalmol. 1958;42:702.
- Knapp A. Optic neuritis after influenza, with changes in the spinal fluid. Arch Ophthalmol. 1916;45:247–249.
- Centers for Disease Control. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010. www.cdc.gov/h1n1flu/cdcresponse.htm. Last accessed July 5, 2011.
- Faridi OS, Ranchod TM, Ho LY, Ruby AJ. Pandemic 2009 influenza H1N1 retinopathy.Can J Ophthalmol. 2010;45(3):286–287.
- Lai CC, Chang YS, Li ML, Chang CM, Huang FC, Tseng SH.. Acute anterior uveitis and optic neuritis as ocular complications of influenza a infection in an 11-year-old boy.J Pediatr Ophthalmol Strabismus. 2011;48:e30–e33.
- Centers for Disease Control: What to Do If You Get Sick: 2009 H1N1 and Seasonal Flu. www.cdc.gov/h1n1flu/sick.htm. Last accessed April 18, 2011.
- Marin J, Kese D, Potocnik M, Butina R.. Laboratory diagnosis of herpesviruses.Acta Dermatovenerol APA. 2000;9:1–7.
- Fekkar A, Bodaghi B, Touafek F, et al. Comparison of immunoblotting, calculation of the Goldmann-Witmer Coeffecient, an real-time diagnosis for ocular histoplasmosis. J Clin Microbiol. 2008;46(6):1965–1967.
- Rothova A, de Groot J, Mudrikova T. Reactivation of acute retinal necrosis after flu H1N1 vaccination. Br J Ophthalmol. 2011;95:291.