Abstract
Purpose: To report two cases of patients with ocular manifestations of human T-cell lymphotropic virus type-1 (HTLV-1) associated adult T-cell leukemia/lymphoma (ATL) who were successfully treated with interleukin-2 receptor targeted therapies.
Method: Case series.
Results: Two patients with HTLV-1-associated ATL developed symptomatic scleritis. In the first case, conjunctival biopsy showed leukemic infiltration that was confirmed by T-cell receptor polymerase chain reaction (PCR) demonstrating a clonal rearrangement. As treatment for ATL, both cases received interleukin-2 receptor targeted therapy. In one patient, daclizumab, a monoclonal antibody directed against the alpha chain of the interleukin-2 (IL-2) receptor, was used. The second patient was treated with denileukin diftitox, an immunotoxin fusion protein that targets the IL-2 receptor. Improvement in scleritis was noted in both patients.
Conclusion: Scleritis in patients with underlying HTLV-1-associated ATL is responsive to IL-2 receptor targeted therapies.
Scleritis is an uncommon ocular disease often associated with rheumatologic disorders and rarely with an infectious or malignant etiology. Its pathogenesis is not well understood and in noninfectious cases is thought to be immune mediated. Human T-cell lymphotropic virus type-1 (HTLV-1) is a retrovirus and the first virus found to be a causative agent of a human malignancy, adult T-cell leukemia/lymphoma (ATL). Worldwide, approximately 10–20 million people are infected with HTLV-1. Transmission occurs primarily through breast-feeding, and less through sexual contact or sharing contaminated needles. The virus exhibits a tropism for CD4+ T cells and requires cell–to–cell contact for transmission.Citation1 In HTLV-1-infected individuals, uveitis is the most common ocular manifestation reported. Other manifestations include episcleritis, scleritis, keratoconjunctivitis, leukemic and lymphomatous infiltrates, papilledema, retinal vasculitis, and choroidopathy.Citation2 HTLV-1infected T cells are known to produce a variety of pro-inflammatory cytokines, including IL-2, without any stimuli. Therefore, ocular inflammation associated with HTLV-1 can be due to infiltration or secondary inflammation. Information on IL-2-targeted therapy in scleritis or HTLV-1-associated ocular disease is scarce. Herein, we describe 2 cases of HTLV-1-associated ocular disease successfully treated with interleukin-2 receptor-targeted therapies.
CASE 1
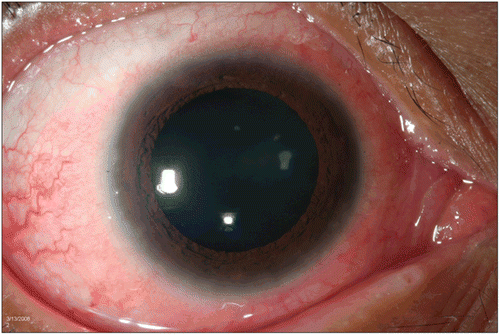
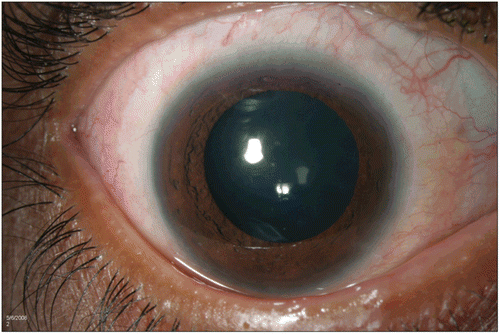
A 15-year-old Hispanic male presented with a 3-day history of “red” eyes. One year earlier he was diagnosed with HTLV-1-associated ATL and treated with a chemotherapy regimen consisting of etoposide, doxorubicin, vincristine, prednisone, and cycolophosphamide (EPOCH). His visual acuity was 20/20 with an anterior segment exam remarkable for bilateral 2+ conjunctival hyperemia, which remained after topical phenylephrine, follicles, and a limbal keratitis. No anterior or posterior chamber inflammation was observed. Conjunctival biopsy showed leukemic infiltration confirmed by demonstrating a monoclonal T-cell receptor rearrangement by PCR. The patient was treated with intravenous daclizumab (Zenapax, Roche Pharmaceuticals, Nutley, NJ) 8 mg/kg every 3 weeks on an investigational study. Two weeks after the first dose of daclizumab, scleral injection was decreased and by 2 months there was marked improvement in the patient’s eye findings ( and ). Treatment was later discontinued because the underlying systemic disease failed to respond.
CASE 2
A 55-year-old Jamaican female presented with a 1-week history of red eyes and left eye pain during a relapse of HTLV-1-associated ATL, diagnosed 1 year earlier and initially treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). On presentation, visual acuity was 20/25 OU. Topical 10% phenylephrine resulted in 1+ and 2+ scleral injection in the right and left eyes, respectively. No anterior chamber or vitreous inflammation was noted. During presentation, the patient was in a clinical trial for ATL and treated with the immunotoxin denileukin diftitox (Ontak, Ligand Pharmaceuticals, La Jolla, CA) at a dose of 9 µg/kg/day for 5 days every 2 weeks. During treatment, the scleritis resolved within 2 weeks.
DISCUSSION
Both agents used to treat HTLV-associated ATL work by targeting the interleukin 2 receptor (IL-2R) and thus interrupting the T-cell immune response. IL-2R is known to be overexpressed in HTLV-1-associated ATL, autoimmune diseases, and allograft rejection and is not normally expressed on resting cells. This provides a valuable target for immunotherapy. Daclizumab, a humanized monoclonal antibody, binds to the IL-2R alpha subunit, blocking the interaction between IL-2 and its receptor leading to cytokine deprivation-mediated apoptotic cell death.Citation3 Denileukin diftitox, however, is a fusion protein that binds to IL-2R and, once internalized, releases diphtheria toxin, which inhibits protein synthesis and induces cell death. Both agents have been used with some efficacy in patients with HTLV-1-associated ATL and in autoimmune disease.Citation4 They inhibit proliferation or eliminate T cells expressing IL-2 receptors. For treatment of ocular inflammatory disease, daclizumab has already been used for uveitis, but its use in scleritis has not been fully explored. In fact, there are only 2 cases where daclizumab was used for the treatment of scleritis.Citation4 Denileukin diftitox has had some effect in rheumatoid arthritis and psoriasis.Citation5,Citation6.
Scleritis is also thought to be a T-cell-mediated disease with increased HLA-DR and IL-2R expression. In these two cases, improvement in ocular disease was secondary to either treatment of the underlying malignancy or the inflammatory response. However, the speed with which the improvement is observed leads us to believe it was the latter, especially since both patients’ systemic disease later relapsed. IL-2 receptor directed therapy may therefore be a treatment option for scleritis mediated by activated or leukemic T cells.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007:7(4):270–280.
- Buggage RR. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr Opin Ophthalmol. 2003:14(6):420–425.
- Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol. 2007:27(1):1–18.
- Papaliodis GN, Chu D, Foster CS. Treatment of ocular inflammatory disorders
- with daclizumab. Ophthalmology. 2003 Apr;110(4):786–789.
- Manoukian G, Hagemeister F. Denileukin diftitox: a novel immunotoxin. Expert Opin Biol Ther. 2009:9(11):1445–1451.
- Yeh S, Wroblewski K, Buggage R, et al. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. J Autoimmun. 2008:31(2):91–97.