Abstract
We describe the clinicopathologic findings in a case of acute rejection following cultivated limbal stem cell transplantation (CLSC). This is the first time lymphangiogenesis has been described in CLSC rejection. This indicates that 1. absence of corneal stromal cells or 2. down regulation of HLA antigens by exposure to culture conditions, does not reduce the risk of graft rejection when transplanting into a high risk corneal bed. Our findings highlight the need for development of anti-lymphangiogenic treatments prior to CLSC transplantation.
Sir,
A 45-year-old male presented at our clinic with a red eye, decreased visual acuity, and mild photophobia. There was a history of chemical burn of the left eye followed by two failed penetrating keratoplasties (PK). On examination the corneal graft in situ appeared opaque with vascularization and an inferior corneal epithelial defect. There was an absence of limbal palisades of Vogt, and corneal reflex was decreased. Schirmer’s test was normal. Best-corrected visual acuity was less than 0.013.
Impression cytology of the cornea showed positive staining with cytokeratin 19 and negative staining with cytokeratin 3/12, confirming 360 degree conjunctivalization and limbal stem cell deficiency (, ). The patient, despite having unilateral involvement, did not consent to donate tissue from his good eye, which had a history of keratoconus with presence of endothelial guttata. In the absence of living related donors, the consensus was to take a limbal biopsy from a cadaveric eye from the cornea bank. A cadaveric cultured limbal epithelial stem cell transplant was preferred over a keratolimbal allograft (KLAL) since the patient was considered at high risk for rejection (young with history of 2 prior vascularized PKs) and there is evidence to suggest that antigenicity of the graft is reduced during culture.Citation1 The protocol was approved by the hospital ethics committee (approval number: EC7/28/153; EudraCT no. 2008-001543-19) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained after explanation of the nature and possible consequences of the procedure.
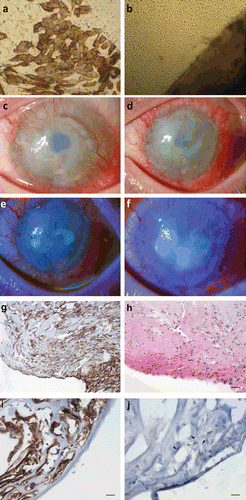
FIGURE 1 (a) Impression cytology of cornea showing positive staining for cytokeratin 19, indicating a conjunctival cell phenotype. (b) Confocal microscopy of epithelial cell outgrowth from the biopsy (lower right) at day 12 in culture. (c) Preoperative photograph showing 360° limbal stem cell deficiency and conjunctivalization of the cornea. (d) Fifteen days post stem cell transplant, there is limbal injection with corneal edema and superficial vascularization of the graft. Eighteen days postoperatively epithelial defects become visible (e), which coalesce to form one large defect by 1 month postoperatively (f). (g) Immunohistochemistry of removed corneal pannus with anti-D2-40 a lymphocyte endothelial marker showing staining around numerous lumen-like structures within the corneal stroma, 200× magnification. (h) Serial section of removed corneal pannus with hematoxylin and eosin stain showing infiltration of numerous neutrophils into the graft, 200× magnification. (i) Arrowheads indicate lymph vessel lumen with lymphatic endothelium staining positive for anti-D2-40 antibody, 400× magnification. (j) Arrowheads indicate lymph vessel lumen with negative staining with anti-CD31, 400× magnification. Scale bars in (g) and (h) represent 100 and 50 µm in (i) and (j).
A superficial limbal biopsy 3 × 3 mm and up to 100 μm deep was removed from a cadaveric donor eye, rinsed with culture medium CNT-20 (CellnTec; Bern, Switzerland) containing antibiotics, and placed epithelial side up onto the basement membrane of a standardized (thermolysin-treated, spongy layer removed) amniotic membrane as previously reported.Citation2 The culture medium, CnT-20 with 1% human AB serum, was refreshed every 2–3 days. The limbal epithelial cells showed an outgrowth of 12.5 mm by day 11 and the composite graft was ready for transplantation ().
The surgery was performed under general anesthesia using our previously published “no-touch surgical technique.”Citation2 Briefly, a 360° conjunctival periotomy was performed at the limbus and all fibrovascular pannus removed. Fibrin glue was applied to the center of the cornea, and the graft, present in the amnion ring, was positioned centrally and tapered to the recipient bed. The graft was covered with Helon (Abbot-Advanced Medical Optic, Santa Ana, CA) and a second amniotic membrane was used as a temporary patch over the graft and sutured to the conjunctiva at 4 cardinal points. A bandage contact lens was applied postoperatively and left in place until the sutures were removed at day 15.
The patient was prescribed the following regimen: 0.3% ofloxacin drops 4×/day, prednisolone drops 8×/day, autologous serum drops 8×/day, oral cyclosporin A 2 × 125 mg/day (commenced 1 week prior to transplantation maintaining blood trough levels at approx. 100–150 ng/mL), and Solu Medrol 125 mg iv perioperatively and continued for 3 days postop and switched to methylprednisolone 1 mg/kg/day.
There were no complications reported during surgery. The patient complained of pain in the eye on day 2 postoperatively. Sutures securing the amniotic membrane patch were removed on day 15 along with the patch. The patient complained of photophobia and pain. There was limbal injection along with corneal edema despite local and systemic immunosuppression (). Two small central epithelial defects emerged, which coalesced to form one large defect despite use of autologous serum drops and soft bandage contact lens (). By 1 month post stem cell transplant, there was limbal injection, heavy corneal neovascularization, irregular epithelium with persistent defects, haze, and edema; the graft was deemed a failure.
The patient agreed to a second stem cell transplant and this time consented to donate a small biopsy from his right eye. The procedure was repeated 4 months later, with the autologous cultivated stem cell graft, and the removed pannus (failed stem cell graft) was sent for pathology. On hematoxylin and eosin staining, numerous neutrophils were seen within the pannus (). On immunohistochemistry, multiple irregular lumen-like structures were observed and anti-D2-40 antibody, which is a specific lymphatic endothelial marker, showed positive staining (brown reaction product) of the cells lining the lumen (). These vessels were thin-walled, collapsed, and irregular with an absence of red blood cells in their lumen. These cells lining the vessel walls stained negative for anti-CD31, which is a vascular endothelial marker specific for blood vessels, confirming their lymphatic nature (). D2-40 expression in systemic lymphatic vessel endothelium served as a positive control. The donor site healed completely, but the patient presented at the emergency department 3 days later with a perforation at the PK donor–acceptor junction of the stem cell recipient eye. An urgent PK was performed, but failed shortly thereafter. Visual acuity was reduced to counting fingers at 2 m.
Cultivated limbal epithelial stem cell transplantation has lower rates of rejection compared with KLAL and less stromal scarring,Citation1 and therefore was given preference in this high-risk case. There is evidence in the literature to show that limbal epithelial cells that are subjected to culture conditions result in a downregulation of their HLA-DR expression and hence a reduction in antigenicity.Citation1 However, despite systemic immunosuppression, the graft showed clinical signs of failure. In the absence of infectious or neurotrophic causes and with the presence of inflammatory infiltrates, lymphatics, and blood vessels on histopathology, the most plausible reason for failure was acute rejection (<4 weeks). Keeping in mind that there was a prior history of failed corneal transplants, there may already have been preformed antibodies present to some MHC (major histocompatibility complex) antigens and present at the time of the stem cell transplant. Presence of lymph vessels in the failed graft supports immunological rejectionCitation3 (the normal cornea is devoid of lymph vessels): lymphatics complete the afferent arm of the immune reflex and enable access of antigens and antigen-presenting cells to the regional lymph nodes where accelerated sensitization to graft antigens occur. The blood vessels provide the efferent arm and sensitized T-cell access to the transplant with resulting graft cell lysis or production of cytokines that recruit other inflammatory cells, eventually causing breakdown of the allograft tissue.Citation4
Only recently Dietrich et al. have described lymphatic vessels as the primary mediators of immune rejection after transplantation and not blood vessels as was previously thought.Citation5 Recent studies are beginning to highlight the importance of lymphatics in acute transplant rejection, primarily because of the recent introduction of specific lymphatic endothelial markers such as D2-40.Citation3,Citation6-11 To our knowledge this is the first time lymphangiogenesis has been described in cultivated limbal epithelial stem cell transplant rejection. This indicates that (1) absence of corneal stromal cells or (2) downregulation of HLA antigens by exposure to culture conditions does not reduce the risk of graft rejection when transplanting into a high-risk corneal bed.
There may be implications with respect to the future of allogenic cultivated limbal stem cell transplantation in high-risk total limbal stem cell deficiency. Since lymphatics are clinically invisible with the slit-lamp examination,Citation12 their presence in high-risk corneas may be underperceived. Recent studies using in vivo confocal microscopy of presumed lymphatic vesselsCitation13;Citation14 are a step toward their clinical detection in transplant rejection.
Dissection of the corneal pannus may not necessarily remove all fibrovascular or lymphatic tissue predisposing the graft bed to high risk of graft rejection (body of evidence suggests new lymphatics stem from preexisting onesCitation15). This underscores the need for developing pretreatment modalities for high-risk graft beds with anti-lymphangiogenic therapeutic agents, such as bevacizumab,Citation8 to improve chances of graft survival.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Oh JY, Ko JH, Lee HJ, Kim MK, Lee JH, Wee WR. The antigenicity of ex vivo cultivated human corneal limbal epithelial and stromal cells: temporal changes in vitro. Cornea. 2010;29:1302–1307.
- Zakaria N, Koppen C, Van TV, Berneman Z, Hopkinson A, Tassignon MJ. Standardized limbal epithelial stem cell graft generation and transplantation. Tissue Eng Part C Methods. 2010;16:921–927.
- Ling S, Qi C, Li W, Xu J, Kuang W. Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin Exp Ophthalmol. 2009;37:874–883.
- Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol. 2009;24:135–138.
- Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539.
- Chang LK, Garcia-Cardena G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–11663.
- Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050.
- Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673.
- Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815.
- Pedica F, Ligorio C, Tonelli P, Bartolini S, Baccarini P. Lymphangiogenesis in Crohn’s disease: an immunohistochemical study using monoclonal antibody D2-40. Virchows Arch. 2008;452:57–63.
- Regina M, Zimmerman R, Malik G, Gausas R. Lymphangiogenesis concurrent with haemangiogenesis in the human cornea. Clin Exp Ophthalmol. 2007;35:541–544.
- Cursiefen C, Schlotzer-Schrehardt U, Kuchle M et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135.
- Peebo BB, Fagerholm P, Lagali N. In vivo confocal microscopy visualization of presumed lymph vessels in a case of corneal transplant rejection. Clin Exp Ophthalmol. 2011;39:832–834.
- Messmer EM, Mackert MJ, Zapp DM, Kampik A. In vivo confocal microscopy of normal conjunctiva and conjunctivitis. Cornea. 2006;25:781–788.
- Bruyere F, Noel A. Lymphangiogenesis: in vitro and in vivo models. FASEB J. 2010;24:8–21.