Giant cell arteritis (GCA) is a systemic vasculitis of the elderly affecting large and medium-size cranial arteries.Citation1 Late recurrent ischemic optic neuropathy (ION) in GCA is not common. Recurrent symptoms generally include malaise, weight loss, myalgia, headache, or scalp tenderness.Citation2 The oculomotor nerve palsy in GCA may be self-limited and respond to corticosteroid treatment.Citation3 We report for the first time the combination of recurrence of bilateral optic neuropathy and unilateral third cranial nerve palsy in a case of histologically proven GCA.
A 68-year-old man with sudden onset of painless visual loss in both eyes for 5 days was referred to us in 2008. He had suffered from scalp tenderness and headache. The right vision deteriorated rapidly in 2 days. Visual acuity in the left eye decreased the day before he was admitted. Visual acuities were 1/10 on the right and 2/10 on the left. Ophthalmoscopic examination revealed pale swelling of both optic discs. Superficial temporal arteries were hardened with absent pulsation on the right and reduced pulsation on the left. Laboratory investigations revealed an erythrocyte sedimentation rate (ESR) of 96 mm/h. A biopsy of the right superficial temporal artery revealed fragmentation of the internal elastic lamina. The vascular wall was infiltrated with lymphocytes and giant cells. The patient was treated with 1 mg/kg/day oral prednisolone. The symptoms resolved rapidly and visual acuities improved to 2/10 to 3/10, respectively.
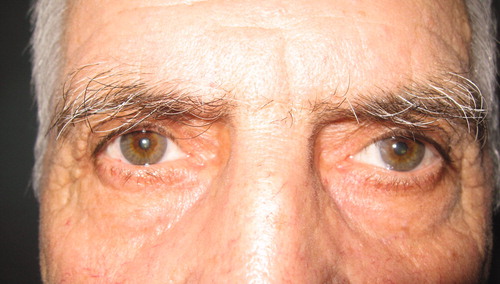
Four years later, the patient was admitted to us with a 4-week history of pain on his scalp and in the occipital area with bilateral acute vision loss and ptosis of the left eye. He complained of cloudy vision in both eyes. He had also experienced weight loss and fatigue during the last months. Ocular examination revealed a visual acuity of 1/10 in the right eye and perception of light with correct light projection in the left eye. The left pupil measured 6 mm in dim illumination and did not constrict to direct light. The left upper eyelid had 2 mm of ptosis (). In the left eye, abduction was normal, but adduction, supraduction, and infraduction were absent. Ophthalmoscopy of the both eyes revealed pale optic discs. The ESR was 69 mm/h (normal value <15 mm/h), and C-reactive protein (CRP) was 8.2 mg/L (normal value <6 mg/L). Neurological examination and brain MRI was found to be normal.
We started with a 3-day induction dose of iv methylprednisolone, 1 g/day, followed by oral prednisone maintenance therapy at an initial dose of 1 mg/kg/day. Three weeks later, the features of third cranial nerve palsy had disappeared except for a dilated unreactive pupil. The visual loss improved to 2/10 and counting fingers at 5 m, respectively. At the seventh month, the patient is still being treated with 10 mg/day oral prednisolone with monthly monitoring of ESR and CRP levels.
Late recurrence of ION and oculomotor nerve palsy are rarely seen in patients with GCA. Liu et al.Citation4 reported recurrent ION in the same eye in 2 patients after initiation of prednisone therapy in a series of 45 patients with GCA. Calamia and HunderCitation5 described a recurrent ipsilateral visual loss 5 months after initiation of treatment.
Bilateral recurrent visual loss and unilateral oculomotor nerve palsy with pupillary involvement occurred in our patient. Pupillary sparing is the hallmark of an ischemic oculomotor palsy, as seen classically in patients with diabetes. In oculomotor palsy in GCA, the pupil is usually spared due to the peripheral position of the pupillary nerve fibers in the oculomotor nerve. However, in our patient there was pupillary involvement, which may be caused by a more severe damage to the nerve than usual. There was malaise and temporal headache, and acute phase reactants were found to be elevated.
Although late recurrence of arteritic ION is rare, our case emphasizes that it may occur even in both eyes with pupillary involved oculomotor nerve palsy. We must monitor acute phase reactants closely, watching for an elevation in acute phase reactants or a relapse in systemic symptoms typical of GCA, which may alert us to a recurrence of ION with or without oculomotor nerve palsy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aiello PD, Trautmann JC, McPhee TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100:550–555
- Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis: trends and clinical spectrum in 161 patients. Medicine. 2000;79:283–292
- Barricks ME, Traviesa DB, Glaser JS, et al. Ophthalmoplegia in cranial arteritis. Brain. 1977;100:209–221
- Liu GT, Glaser JS, Schatz NJ, et al. Visual morbidity in giant cell arteritis: clinical characteristics and prognosis for vision. Ophthalmology. 1994;101:1779–1785
- Calamia KT, Hunder GG. Clinical manifestations of giant cell (temporal) arteritis. Clin Rheum Dis. 1980;6:389–403