Abstract
Purpose: To present a case of autoimmune retinopathy resulting from a mutation in the autoimmune regulator (AIRE) gene.
Design: Case Study
Methods: Case Review
Results: Mild improvement of goldmann visual field following treatment with systemic and local immunosuppression.
Conclusions: There are a limited number of cases linking autoimmune retinopathy with a mutation in the AIRE gene. Further research is needed to find more effective treatment and to prevent tissue damage.
A 19-year-old woman noted progressive nyctalopia, difficulty in dark adapting, and intermittent photopsias for 3 weeks. Her medical history includes an idiopathic stroke at age 15 months and autoimmune polyendocrine syndrome (APS) type I (hypoparathyroidism at 4 years and Addison disease at 15 years), with two disease-associated autoimmune regulator (AIRE) gene mutations (R257X and 967_979del13) in compound heterozygosity.Citation1–3 Her medications included prednisone 5 mg, divalproic acid, calcitriol, and aspirin.
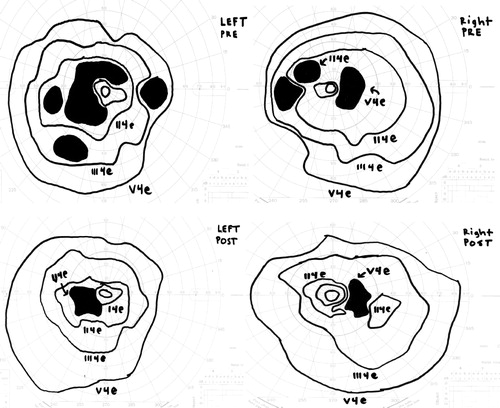
On presentation her best-corrected visual acuity was 20/25 OU. Pupils were normal. She identified 8/10 Ishihara color plates, right eye; and 5/10, left eye. Slit-lamp exam showed 1+ anterior vitreous cells OU. Dilated fundus exam appeared normal except for moderately attenuated vessels, and fundus autofluoresence demonstrated a perifoveal ring of hyperfluorescence, indicating RPE dysfunction (). SD-OCT showed perifoveal attenuation at the inner segment/outer segment junction of the photoreceptors OU (). Fluorescein angiography was normal. Full-field and multifocal electroretinograms (ERG) were abnormal, suggesting severe diffuse photoreceptor damage (). Goldmann visual fields (GVF) showed midperipheral scotomas ().
Figure 1. Autofluorescence showing a perifoveal ring of hyperfluorescence, suggestive of RPE dysfunction.
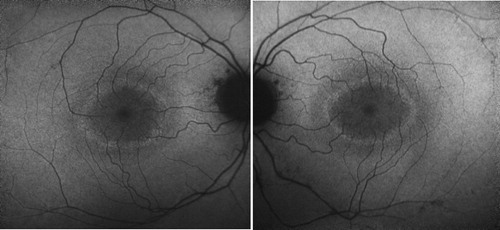
Figure 2. OCT reveals attenuation of the perifoveal inner segment/outer segment junction of both eyes.
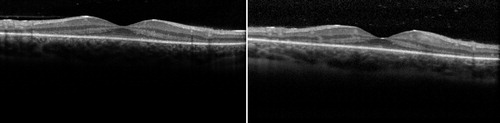
Figure 3. (Top) Results of multifocal ERG. Each image shows the patient's results on the left compared to normal control on the right. Note severe flattening of the mf ERG in both eyes. (Bottom) The full-field ERG revealed undetectable maximal rod responses and severely reduced 30 Hz flicker amplitudes in both eyes, indicating rod and cone photoreceptor loss.
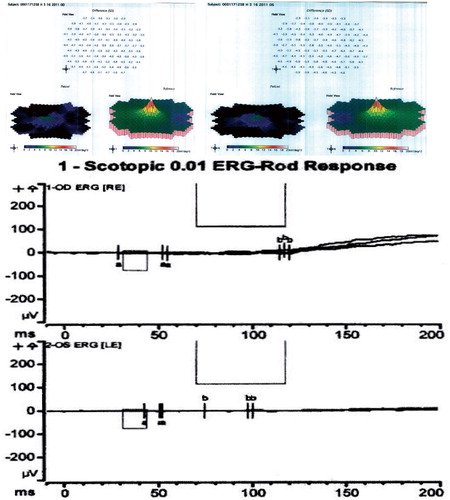
Figure 4. (Top) Pretreatment Goldmann visual field showing enlarged blind spot and midperipheral scotomas in both eyes. Left eye reveals dense superior and inferior arcuate scotomas. (Bottom) Improvement following 11 months of treatment.
The differential diagnosis includes autoimmune retinopathy, cancer-associated retinopathy, retinitis pigmentosa, and other infectious or inflammatory conditions. Workup for inflammatory and neoplastic conditions was negative, including Lyme titer, ANA, ACE, vitamins A and B12, NMO-IgG, syphilis, and brain MRI. Anti-retinal antibody testing by western blot was positive for enolase, a 36-kDa (GAPDH), and a 44-kDa protein.
After discussing the treatment options, the patient elected observation. Three months later her VA was stable, but the GVF appeared slightly worse. She began oral cyclosporine 100 mg and oral prednisone 40 mg with a slow taper, in addition to intravitreal triamcinolone injections. A repeat anti-retinal antibody profile 3 months later demonstrated two new antibodies of unknown significance (50 and 62 kDa), and we added mycophenolate mofetil 250 mg to her therapeutic regimen. We discontinued the intravitreal triamcinolone injections because the patient developed ocular hypertension. Five months later, she denied any changes, her visual acuity remained 20/25 OU, and she was able to identify 10/10 Ishihara color plates. Her GVF showed improvement () and her ERG and OCT remained stable. Subsequent anti-retinal antibody testing did not detect the 50- and 62-kDa antibodies; however, enolase and GAPDH persisted.
To our knowledge, this case represents the first report of autoimmune retinopathy associated with an AIRE mutation and APS. APS is a rare, autosomal recessive disease caused by an AIRE gene mutation located on chromosome 21. There are at least 60 known mutations in AIRE that may cause APS.Citation4 AIRE is a transcription factor that regulates expression of self-antigens in the thymus, and in its absence, autoantibody production and tissue destruction occur.Citation4,Citation5 The classic triad of APS includes mucocutaneous candidiasis, hypoparathyroidism, and Addison disease.Citation5 Ocular features that have been previously described include aqueous tear deficiency, keratoconjunctivitis, corneal neovascularization, hypotrichosis, lens opacities, iridocyclitis, and optic atrophy.Citation6
Anderson et al. used AIRE-knockout mice to show that AIRE regulates thymic gene expression and induction of self-tolerance.Citation7 These mice develop T-cell lymphocytic infiltration of the retina, pancreas, stomach, ovary, and salivary glands, as a result of loss of thymic expression of the interphotoreceptor retinoid-binding protein (IRBP).Citation8 Given these findings, T-cell-mediated infiltration in the retina may play a possible role in the mechanism of AIR in our patient.
Although no definitive treatment for AIR exists, the underlying cause is usually the first target. In APS, patients are typically given hormone replacement and systemic immunosuppression, including cyclosporine, methylprednisolone, mycophenolate mofetil, or methotrexate.Citation6 Case reports and retrospective studies suggest potential benefits of systemic immunosuppression, intravitreal triamcinolone, and intravenous immunoglobulin for AIR associated with active inflammation or cystoid edema.Citation9,Citation10 We suggest that patients may be followed with serial ERG, GVF, OCT, and repeat anti-retinal antibody testing for disease monitoring.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Support for this study was provided by an unrestricted grant from Research to Prevent Blindness, New York, NY (SM, MSL).
References
- Nagamine, K, Peterson, P, Scott, HS, et al. Positional cloning of the APECED gene. Nature Genet. 1997;17:393–398
- Aaltonen J, Björses P, Perheentupa J, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nature Genet. 1997;17:399–403
- Heino M, Peterson P, Kudoh J, et al. APECED mutations in the autoimmune regulator (AIRE) gene. Hum Mutat. 2001;18:205–211
- Akirav EA, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol. 2011;7:25–33
- Gardner JM, Fletcher AL, Anderson MS, et al. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21:582–589
- Chang B, Brosnahan D, McCreery K, et al. Ocular complications of autoimmune polyendocrinopathy type I. J AAPOS. 2006;10:515–520
- Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self-shadow within the thymus by the AIRE protein. Science. 2002;298:1395–1401
- DeVoss, J, Yafei, H, Johannes, K, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735
- Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;30:127–134
- Ferreyra HA, Jayasundera T, Khan NW, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127:390–397
